4349
Markov Model of Lung Cancer Screening Demonstrates Equivalent Lung Cancer Detection using either Lung MRI or Low-Dose CT Screening Strategies1Radiology, Northwestern University, Chicago, IL, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Diagnostic and Interventional Radiology, University of Heidelberg, Heidelberg, Germany, 4Translational Lung Research Center Heidelberg (TLRC), Member of the German Lung ResearchCenter (DZL), Heidelburg, Germany, 5Radiologie Darmstadt, Darmstadt, Germany, 6Radiation Oncology, Northwestern University, Chicago, IL, United States, 7Medicine - Hematology and Oncology, Northwestern University, Chicago, IL, United States, 8Surgery - Thoracic Surgery, Northwestern University, Chicago, IL, United States, 9Industrial Engineering and Management Sciences, Northwestern University, Evanston, IL, United States
Synopsis
Lung cancer screening with low dose CT (LDCT) has been shown to result in a 20% mortality reduction, but has relatively low specificity for lung cancer diagnosis, as well as concerns related to radiation dose and overdiagnosis. Lung MRI has similar sensitivity and improved specificity for lung cancer detection. In this study, we developed a Markov model of lung cancer screening to compare performance of LDCT and MRI. Based on our analysis, lung cancer screening with MRI could provide an equivalent number of lung cancer diagnoses, while dramatically reducing the number of false positive findings relative to LDCT.
Introduction
Based on findings from the National Lung Cancer Screening Trial (NLST), the United States Preventative Task Force has recommended annual low-dose CT (LDCT) lung cancer screening in high-risk patients. However, there are concerns about increased radiation exposure as well as overdiagnosis of lung cancer associated with LDCT screening, and there were a significant number of false positive screening exams in the NLST. MRI has recently demonstrated modest sensitivity and high specificity for malignant nodules 1 cm or greater in size (1). The purpose of this study was to create a Markov model of lung cancer screening to evaluate the potential performance of lung MRI vs. LDCT. We hypothesized that MRI would have similar sensitivity with improved specificity for diagnosing lung cancer relative to LDCT with annual screening over 20 years.Methods
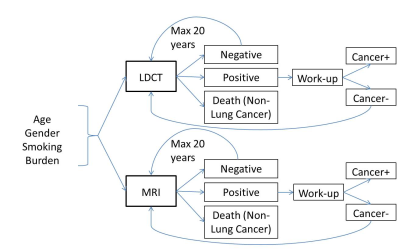
We converted the MISCAN Lung microsimulation (2) of lung cancer progression and clinical diagnosis into a Markov cohort model. In addition, our model used Meza et al. (3) to specify lung cancer incidence based on gender, age and smoking burden (cigarettes per day). As in the MISCAN model, we divided cancers into histologic subtypes (small cell carcinoma, adenocarcinoma, squamous cell carcinoma, and all additional lung/bronchus histologic subtypes). We estimated the probability of subtype incidence by age using NLST data. We used Rosenberg et al (4) to specify background mortality by age excluding lung cancer mortality, and used the Surveillance, Epidemiology, and End Result (SEER) database to extract survival after diagnosis for each combination of histology subtype and stage. To evaluate potential screening performance, we used the model to calculate the probability of true/false positive and true/false negative screening results in high-risk cohorts. We ran this analysis over a horizon of 20 years with annual screening (Figure 1). A patient would leave the screening pool after a true positive test or death, leading to an average number of screening procedures for each analyzed cohort. The time-0 composition of the cohort was a mixture of well and undiagnosed cancer patients in different stages and histologies taken as the equilibrium mixture from the model when run from age 40. The probability of a positive CT screening exam was based on previously reported Sn and Sp by stage and histology.(2, 5) For MRI, there is no data currently available for stage/histologic subtype, so overall Sn/Sp for lung cancer detection was used.(1) Our analysis considers only solid nodules and does not consider CT or MR detection of ground glass nodules. The analysis was run in four separate gender/age cohorts, all with a smoking burden of 40 cigarettes per day: 1) Male/50 years old, 2) Female/50 years old, 3) Male/60 years old, 4) Female/60 years old.Results
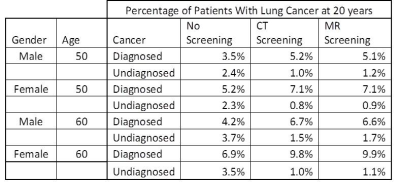
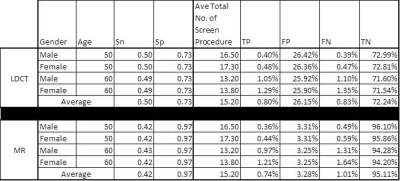
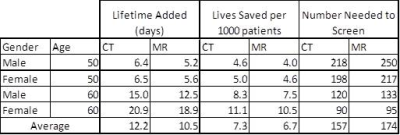
There was no difference in average number of screening rounds between LDCT and MRI. Compared to no screening, both CT and MRI led to increased cancer diagnoses, and there was an essentially equivalent percentage of cancer diagnoses between the two strategies. (Table 1) The percentage of true positives and false positives were higher for CT screening, while the percentage of true negatives and false negatives were higher for MRI. The average Sn/Sp was 0.50/0.73 for CT and 0.42/0.97 for MRI. (Table 2) On average, CT added 12.2 days of life per patient and saved 7.3 lives per 1000 patients screened annually over 20 years (number needed to screen: 157 patients). MRI added 10.5 days of life per patient and saved 6.7 lives per 1000 patients screened (number needed to screen: 174 patients). (Table 3) These findings suggest an approximately 9% relative mortality reduction with CT screening, with an additional .6 in 1000 lives saved over MRI in 20 years of annual screening.Conclusions
Based on our analysis, lung cancer screening with MRI could provide an equivalent number of lung cancer diagnoses, while dramatically reducing the number of false positive findings relative to LDCT. However, there is a slight mortality benefit for CT screening relative to MRI, likely the result of earlier diagnosis with CT due to higher sensitivity. The NLST demonstrated a 20% mortality reduction with LDCT, and our results suggest MRI could be expected to result in an approximately 18% mortality reduction. Ultimately, the reduced false positive rate with MRI should lead to cost savings and reduced complications from the work-up of positive tests. Additional considerations such as the risk of radiation-induced malignancy with CT and the likelihood of CT over diagnosis have not yet been included in these considerations and will be addressed in future studies with our model.Acknowledgements
No acknowledgement found.References
1. Sommer G, Tremper J, Koenigkam-Santos M, et al. Lung nodule detection in a high-risk population: comparison of magnetic resonance imaging and low-dose computed tomography. European Journal of Radiology. 2014;83(3):600-5.
2. Ten Haaf K, van Rosmalen J, de Koning HJ. Lung cancer detectability by test, histology, stage, and gender: estimates from the NLST and the PLCO trials. Cancer Epidemiology, Biomarkers and Prevention. 2015;24(1):154-61.
3. Meza R, Hazelton WD, Colditz GA, Moolgavkar SH. Analysis of lung cancer incidence in the Nurses' Health and the Health Professionals' Follow-Up Studies using a multistage carcinogenesis model. Cancer Causes and Control. 2008;19(3):317-28.
4. Rosenberg MA, Feuer EJ, Yu B, et al. Chapter 3: Cohort life tables by smoking status, removing lung cancer as a cause of death. Risk Analysis. 2012;32 Suppl 1:S25-38.
5. Ten Haaf K, Jeon J, Tammemagi MC, et al. Risk prediction models for selection of lung cancer screening candidates: A retrospective validation study. PLoS Med. 2017;14(4):e1002277.