4343
Examination of Lung Function among Older Smokers with and without COPD by Apparent Diffusion Coefficient (ADC) of 3He MRI1Department of Medicine, Columbia University Medical School, New York, NY, United States, 2Department of Biomedical Engineer, Columbia University, New York, NY, United States, 3Department of Radiology, Columbia University Medical School, New York, NY, United States, 4Department of Chemistry, Lakehead University, Thunder Bay, ON, Canada, 5Department of Infection, Immunity & Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom, 6Department of Physics, Columbia University, New York, NY, United States
Synopsis
Chronic obstructive pulmonary disease (COPD) is defined as persistent airflow limitation by spirometry. However, some smokers with normal spriometry have significant respiratory symptoms. We used 3He apparent diffusion coefficient (ADC) to examine the lungs in older smokers with and without COPD (n=50). This study showed high ADC in both smokers with and without COPD. The difference in ADC between COPD and non-COPD was significant. ADC was correlated positively with percent emphysema and %FVC, and negatively with FEV1 to FVC ratio and, non-significantly with FEV1. 3He ADC may provide different information of lung microarchitecture from spirometry in smoking related pulmonary diseases.
INTRODUCTION
The ill effects of smoking are myriad, including cancer, cardiovascular disease, lung diseases such as chronic obstructive pulmonary disease (COPD). COPD is defined as persistent airflow limitation in smokers according to spirometric assessment1. However, studies have shown that more than 50% of smokers with normal spirometry have respiratory-related symptoms 2,3. Spirometric measurements and CT scans used routinely for diagnosis and evaluation of the severity of COPD have limitations. In recent years, hyperpolarized Helium (3He) MRI has been used to provide physiological insights into lung structure and function4. The inhaled 3He atoms are restricted by the luminal dimensions of bronchioles and alveoli. This restriction can be evaluated as apparent diffusion coefficient (ADC)5-10. The purpose of this study was to use ADC of 3He MRI to examine lung disease among older smokers with and without COPD.METHODS
The local Institutional Review Board approved this Health Insurance Portability and Accountability Act (HIPAA) compliant study. All participants provided written informed consent to the study protocol. All participants (n= 50) aged 60-85 years who had 10 or more pack-years of smoking underwent high resolution chest CT scans, spirometry and 3He MRI. The 50 participants were divided into the non-COPD control group (n=24) or the COPD group (n=26 with 42 % mild and 58% moderate), with an average percentage predicted FEV1 of 101.3 ± 21.4 % and 78.7 ± 14.3%, respectively. Percent emphysema was defined as the percentage of the lung below the attenuation threshold of -950 HU on computed tomography. ADC MRI was performed with a flexible wrap-around 3He RF coil (CMRS) on a Phillips Achieva 3T scanner. A GE spin exchange polarizer was used to polarize the 3He gas with a polarization of 30 ± 6%. Prior to 3He scans, a conventional proton MRI was performed as a survey scan for positioning. 3D diffusion images were acquired without and with diffusion sensitizing gradient with b-values of 0 and 1.6 s/cm2 , respectively. The participant was asked to breath in 1 liter of a 3He gas mixture (300 ml of hyperpolarized 3He gas with 700 ml of nitrogen) from residual volume with instructions to continue to breath in a room air chaser to “top off” the lungs up to total lung capacity (TLC). Semi- automated calculation of ADC was performed using custom Matlab software. ADC color maps and histograms were generated slice by slice. Pearson’s correlation analysis adjusted for age, sex and COPD status was performed using SAS.RESULTS
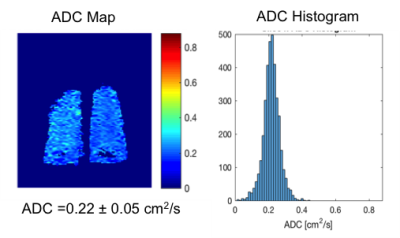
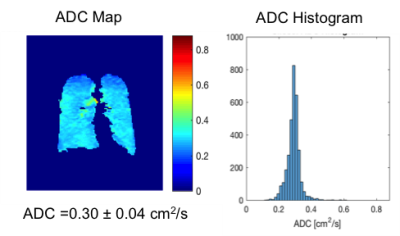
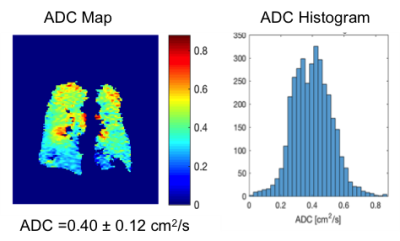
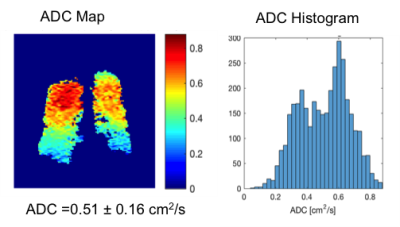
Fig. 1-4 are examples of ADC maps in different disease group. ADC map and histogram analysis showed a homogeneous and narrow distribution in the lungs of a healthy non-smoker with a low mean ADC value of 0.2 cm2 /s (Fig 1), and in smokers without COPD with increased an ADC value of 0.3 cm2 /s (Fig. 2). In contrast, there was inhomogeneity and broadening of the distribution in the COPD group with a high mean ADC value of 0.4 cm2 /s in the mild-COPD (Fig 3) and 0.5 cm2/s in moderate COPD (Fig 4). Areas with higher ADC values shown on the ADC maps represents the parenchymal destruction and inhomogeneous distribution of the disease process. The difference in mean ADC between COPD (ADC = 0.4 cm2/s, n= 26) and non-COPD controls (ADC = 0.3 cm2/s, n=24) was significant (P=0.0005). Among the COPD group, there was an increase in ADC across increasing GOLD categories of COPD severity (P-trend=0.0002). ADC was correlated positively with percent emphysema (r=0.68, p<0.0001) and post-bronchodilator %FVC (r=0.51, p=0.01) and negatively with post-bronchodilator FEV1 to FVC ratio (r=-0.58, p=0.004) and, nonsignificantly, with post-bronchodilator FEV1 (r=-0.21, p=0.34).DISCUSSION AND CONCLUSION
ADC is restricted in acinar spaces from its free diffusivity value in air of 0.88 cm2 /s down to 0.2 cm2 /s in lungs of healthy non-smokers. Our study shows higher ADC values in both older smokers without and with COPD, which is consistent with other studies with COPD, healthy smoker volunteers and ex-smoker 7,9. 3He ADC measures acquired at TLC show high correlation with lung structure on CT and airflow limitation but interestingly, not with COPD severity as assessed by the FEV1. The non-COPD smokers with normal FEV1 in our study have considerable respiratory-related symptoms. The elevated ADC in this group revealed abnormalities of the lungs that correlated with their symptoms. Our and other studies7-10 demonstrated that 3He ADC measurement might be more sensitive to certain effects of smoking than spirometry which can be used to assess lung function in smoking related lung disease.Acknowledgements
NIH/NHLBI R01 HL093081, R01 HL077612References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (updated 2016) (http:// www.goldcopd.org).
2. Woodruff PG, Barr RG, Bleecker E, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med 2016; 374:1811-21.
3. Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med 2015; 175:1539-49.
4. Capaldi DP, Sheikh K, Guo F, Svenningsen S, Etemad-Rezai R, Coxson HO, Leipsic JA, McCormack DG, Parraga G. Free-breathing pulmonary 1H and Hyperpolarized 3He MRI: comparison in COPD and bronchiectasis. Acad Radiol. 2015 Mar; 22(3):320-9.
5. Wild JM, Woodhouse N & Teh K, Single-scan acquisition of registered hyperpolarized (3)He ventilation and ADC images using a hybrid 2D gradient-echo sequence.. Magn Reson Med 2007; 57(6), 1185-1189. 6. Kirby M, Svenningsen S, Owrangi A, Wheatley A, Farag A, Ouriadov A, Santyr GE, Etemad-Rezai R, Coxson HO, McCormack DG, Parraga G. Hyperpolarized 3He and 129Xe MR imaging in healthy volunteers and patients with chronic obstructive pulmonary disease. Radiology. 2012 Nov; 265(2): 600-10.
7. Swift AJ, Wild JM, Fichele S, Woodhouse N, Fleming S, Waterhouse J, Lawson RA, Paley MN, Van Beek EJ. Emphysematous changes and normal variation in smokers and COPD patients using diffusion 3He MRI. Eur J Radiol. 2005 Jun;54(3):352-8.
8. Kirby M1, Owrangi A, Svenningsen S, Wheatley A, Coxson HO, Paterson NA, McCormack DG, Parraga G. On the role of abnormal DL(CO) in ex-smokers without airflow limitation: symptoms, exercise capacity and hyperpolarised helium-3 MRI. Thorax. 2013 Aug;68(8):752-9.
9. Fain SB1, Panth SR, Evans MD, Wentland AL, Holmes JH, Korosec FR, O'Brien MJ, Fountaine H, Grist TM. Early emphysematous changes in asymptomatic smokers: detection with 3He MR imaging. Radiology. 2006 Jun;239(3):875-83.
10. Kirby M1, Heydarian M, Wheatley A, McCormack DG, Parraga G. Evaluating
bronchodilator effects in chronic obstructive pulmonary disease using
diffusion-weighted hyperpolarized helium-3 magnetic resonance imaging. J Appl Physiol
(1985). 2012 Feb;112(4):651-7.