4342
Fast intensity non-uniformity correction for breast MRI using sparse samples1Radiology, Stanford Universty, Palo Alto, CA, United States
Synopsis
The quantitative application of breast MRI is hindered by image non-uniformity artifact due to B0 and B1 variations. In this work, we developed an effective intensity non-uniformity correction algorithm for breast MRI with high computational efficiency. Compare to existing methods, the proposed method is readily implementable clinically as a software plug-in without modification of existing imaging protocols or hardware, and can potentially be applied to MR images of other anatomical sites.
Introduction
Breast MRI images often demonstrate non-uniformity artifact, hindering the quantitative application of breast MRI in tasks that relies on accurate graylevel. Existing approaches for intensity non-uniformity correction can be divided into prospective and retrospective methods. Despite their efficacy in certain conditions, these methods demonstrate different drawbacks due to their limited performances in correction efficacy and computational efficiency[1, 2]. In this work, we propose an effective non-uniformity correction method for breast MRI with negligible processing time, which is highly adaptive to clinical environment.Methods
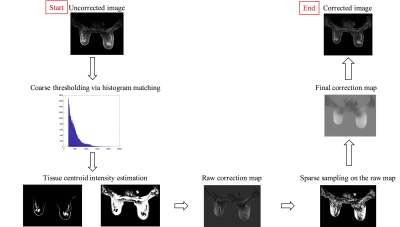
The proposed method (Figure 1) starts with a coarse tissue classification of adipose and glandular tissue via a histogram-based thresholding approach on the images with non-uniformity artifact. The median values of the two tissues are calculated and the intensity differences between the segmented tissue and their corresponding median are considered as the non-uniformity errors, but only sparse samples are selected for correction using empirical constraints to avoid carrying over false information from coarse segmentation results. The sparse sample selection scheme relies on successful removal of outliers in each difference image between tissue and its median. A Fourier-transform based algorithm[3], referred as local filtration, is then used to process the sparse data for obtaining the final non-uniformity correction map. Mathematically, the acquired volumetric non-uniformity correction map,$$$\hat\S_t$$$, can be expressed as:
$$\hat\S_t (i,j,k)=(∑_{(s,t,k)∈Ω_s}S_0 (s,t,u)∙w_σ (i-s,j-t,k-u))/(∑_{(s,t,k)∈Ω_s}w_σ (i-s,j-t,k-u)) \qquad(1)$$
Where the $$$Ω_s$$$ is the sampled region and $$$w_σ$$$ is the Gaussian smooth kernel, defined as:
$$w(s,t,u)=e^{-(s^2+t^2 )/σ_1^2 -u^2/σ_2^2}\qquad\qquad\qquad\qquad(2)$$
Eq (1) is essentially the weighted summation of sampled data, which can be rewritten as
$$\hat\S_t (i,j,k)= ((S_0∙f)\text{***}w)/(f\text{***}w)\qquad\qquad\qquad\qquad(3)$$
Where $$$f$$$ is the binary indication function of the sampled data, Eq (3) can be effectively implemented using fast Fourier transform. The $$$\hat\S_t$$$ is finally removed from the uncorrected images for effective non-uniformity correction.
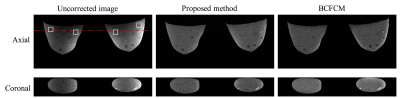
The performance of the proposed method is evaluated on T1-weighted 3D SPGR MR images for a heterogeneous breast phantom (Model 073, CIRS Inc., Norfolk, Virginia) and in Dynamic Contrast Enhanced (DCE) images from 5 patients who underwent abreast MRI research protocol at our institution. All the images (512 x 320 matrix, 27 cm FOV, 1 mm sl thick, 12 sec temporal resolution, pre and peak images 45 sec temporal footprint) are acquired on a GE 3T MR750 scanner (GE Healthcare, Waukesha, WI) using a 16-channel Sentinelle breast coil (Invivo, Gainesville, FL). A comparison study is performed between the proposed method and the bias-corrected FCM(BCFCM) method, which was a modified fuzzy-C-mean(FCM) method previously reported for non-uniformity correction in breast MR images[4, 5]. Spatial non-uniformity (SNU), calculated as the maximum mean difference among four selected regions of interest, and coefficient of variation (CV), defined as the ratio between standard deviation and mean value of the background tissue, are used as quantitative metrics for results evaluation.
Results
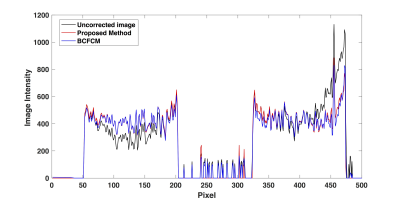
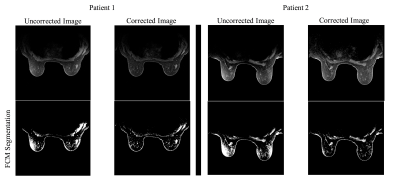
Figure 2 shows the correction results for phantom data using the proposed method and BCFCM. The 1-D profile extracted at the red dashed line in Figure 2 are shown in Figure 3. Both methods effectively remove the non-uniformity artifacts. The SNU (i.e., ROIs in white squares) and CV are reduced from 244 arbitrary unit(AU) and 26% to 19 AU and 2% with proposed method, and 23 AU and 2.3% with BCFCM method. Despite their similar performance on non-uniformity correction, the typical processing time is 3 seconds for an image set of 512x512x199 using the proposed method compared to about 1 min to process one single slice with BCFCM method. Figure 4 shows the correction results for two patients. It is seen that the FCM segmentation accuracy of skin and glandular tissues are substantially improved after correction. The average SNU and CV for 5 patients are reduced from 280 AU to 47 AU and 29% to 5.3% with proposed correction.Discussion and Conclusion
In this work, we developed an effective intensity non-uniformity correction algorithm for breast MRI with high computational efficiency. The method substantially improves the image quality for both phantom and patient MR images with negligible processing time. Such a method is readily implementable clinically as a software plug-in without modification of existing imaging protocols or hardware, and can potentially be applied to MR images of other anatomical sites.Acknowledgements
Research support from GE Healthcare, NIH R01 EB009055 and NIH T32 CA009695References
1. Hou, Z., A Review on MR Image Intensity Inhomogeneity Correction. Int J Biomed Imaging, 2006. 2006: p. 49515.
2. Lin, M., et al., A new bias field correction method combining N3 and FCM for improved segmentation of breast density on MRI. Med Phys, 2011. 38(1): p. 5-14.
3. Shi, L., et al., Fast shading correction for cone beam CT in radiation therapy via sparse sampling on planning CT. Med Phys, 2017. 44(5): p. 1796-1808.
4. Ahmed, M.N., et al., A modified fuzzy C-means algorithm for bias field estimation and segmentation of MRI data. IEEE Trans Med Imaging, 2002. 21(3): p. 193-9.
5. Doran, S.J., et al., Breast MRI segmentation for density estimation: Do different methods give the same results and how much do differences matter? Med Phys, 2017. 44(9): p. 4573-4592.
Figures