4339
RF-Induced Potential False-Negative Lesion in Breast T2-weighted MRI at 3T: Exploration of a Single-Channel kT-Points Solution1CEA/DRF/Joliot/NeuroSpin/UNIRS, Gif-sur-Yvette, France, 2Department of Radiology, AP-HP, CHU Henri Mondor, Créteil, France, 3Siemens Healthcare SAS, Saint-Denis, France, 4Université Paris-Est Créteil Val-de-Marne, Créteil, France, 5INSERM Unité U955, Equipe 18, Créteil, France
Synopsis
Breast MRI can benefit from the improved signal-to-noise ratio brought by high-field systems to achieve finer spatial or temporal resolutions. However, dielectric resonance associated with the shorter RF wavelength provokes inhomogeneous excitation in the tissues. In this work, it was shown that such artefacts can induce hyperintensity in T2-weighted images, thus potentially misleading clinicians into excluding malignancy in a lesion. A solution is proposed to reduce the RF artefact on 3D T2w acquisitions using single-transmit-channel kT-points, which could be used on any 3T scanner.
Introduction
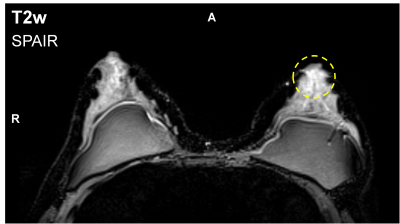
A T2-weighted sequence is often used in conjunction with Dynamic Contrast-Enhanced (DCE) MRI, in order to help differentiating, in some cases, between benign and malignant breast lesions1–4: bright T2 hyperintensity (T2H) can indicate either a cyst, a fibroadenoma or an intramammary lymph node,5 and tends to exclude malignancy. In the last two cases, contrast enhancement can mimic that of malignant lesions. 3T MRI is widely recognized for its potential gain in signal-to-noise ratio, which can be used to improve spatial or temporal resolution. However, this gain can be hampered by locally inhomogeneous excitation due to dielectric resonance as the radiofrequency (RF) wavelength is shortened6. In this work, T2H clearly identified as related to B1+ inhomogeneity is reported, which could lead to false-negative interpretation (Figure 1). Solutions exist to reduce B1+ artefacts, such as dual-transmit RF systems6,7 or the use of dielectric pads7, but require either extra hardware or a dedicated setup. A method was proposed to address the problem in DCE-MRI sequences8, but not in T2-weighted imaging. This study first aims at analysing the impact of excitation inhomogeneity on T2-weighted acquisition breast imaging. Then, the kT-points9,10 pulse design method is proposed to reduce potential B1+ artefacts, with no need for parallel transmission (pTX).Methods
Acquisitions were carried out on a single-transmit-channel MAGNETOM Verio 3T scanner equipped with TrueForm11 (Siemens Healthcare, Erlangen, Germany). An axial T2-weighted three-dimensional SPACE12,13 sequence (TR= 2800ms, TEeffective= 251ms, TEapparent= 90ms, ETL= 100, 256×256x104 matrix, 1.4×1.4×2.0mm3 resolution) with spectral adiabatic inversion recovery fat suppression was used. An occurrence of RF-related T2H has been seen on a 48-year-old subject (BMI= 21.3); T2H in the left breast is highlighted in Figure 1.
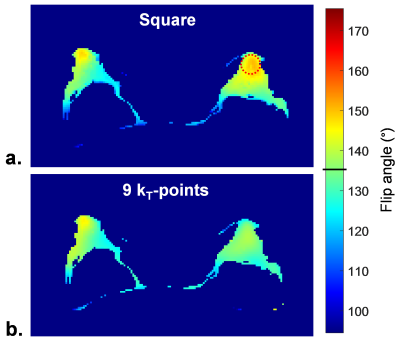
In order to estimate the contribution of RF inhomogeneity to this T2H, flip angle (FA) map simulations based on B1+ and Δf0 (Larmor frequency offset) maps acquired in vivo were performed via numerical integration of Bloch's equation. A FA map was estimated for the largest FA targeted in the refocusing train. SPACE signal simulation was run in every voxel by calculating magnetization rotations successively induced by each refocusing pulse in the train. Uniform breast tissue relaxation times were considered: T1/T2= 1500/50ms.14 The B1+ field map was acquired using a magnetisation-prepared turbo fast low angle shot sequence,15 at a 3x3x4mm3 isotropic resolution. The Δf0 map was acquired with a 2mm isotropic resolution. Three echoes were needed to extract both Δf0 and water/fat information, acquired over two 3D GRE sequences, with Dixon16 reconstruction (TE1/TE2/TE3= 1.23/2.10/2.46ms); Δf0 was calculated with the phase images from TE2 and TE3, using leverage from TE1 for more precision.
Finally, a Δf0-robust scalable 9-kT-point pulse was designed, with MATLAB’s built-in active-set algorithm, under energy and hardware constraints17–19 using Average Hamiltonian Theory.20 This tailored pulse was used for the 90° excitation (with a π/2 phase offset), and for all refocusing pulses20 (FA= 16° to 135°), which allowed a very quick pulse design (less than one minute). SPACE pulse optimisation was restricted to water voxels, as fat-selective saturation is used in the sequence. Simulated FA and signal were compared with those stemming from the default square pulse.
Results
Figure 2a shows the simulated FA map corresponding to the slice pictured in Figure 1. A zone of local FA overshoot (red circle) aligns with the highlighted T2H from Figure 1. Table 1 gathers FA and signal homogeneity results. The relative difference between the mean signal in a 20mm-diameter sphere centred on the left breast FA overshoot region and the mean signal in both breasts is reported in the rightmost column. RF inhomogeneity alone is accountable for a 12.6% T2H. Figure 2b and Table 1 report the FA map simulation for the kT-points pulse. KT-points substantially lower FA normalised root-mean-square error (NRMSE). Most importantly, this would translate into reduced RF-induced T2H.Conclusion
These results point out potential misinterpretation in T2-weighted breast MRI due to RF inhomogeneity at 3T: even if the reported T2H is not solely RF-induced, the artefact could bring a lesion away from a malignancy threshold, thus implying a false-negative diagnosis. Given how not straightforward the SPACE signal is,13 here RF artefacts cannot be removed by post-processing. However, simulations showed that single-channel kT-points pulses, which can be implemented on any scanner, are promising in reducing RF-related inhomogeneity beforehand. They could of course benefit from, or even require pTX to enhance homogeneity sufficiently. Better anatomy-specific masking could also help improving the optimisation. An ongoing study aims at assessing single-channel kT-points in the breasts with actual acquisitions on a variety of subjects, for both T1-weighted DCE-MRI and T2-weighted imaging.Acknowledgements
The authors wish to thank all the MRI technicians of Henri Mondor Hospital for their patience, understanding and helpful clinical advices. This project was funded by CEA’s Programme Transversal, Technologies pour la Santé (Transversal Programme for Health Technologies).References
1. Azlan CA, Di Giovanni P, Ahearn TS, Semple SIK, Gilbert FJ, Redpath TW. B1 transmission-field inhomogeneity and enhancement ratio errors in dynamic contrast-enhanced MRI (DCE-MRI) of the breast at 3T. J Magn Reson Imaging. 2010 Jan 1;31(1):234–9.
2. Moran CJ, Hargreaves BA, Saranathan M, Lipson JA, Kao J, Ikeda DM, et al. 3D T2-weighted spin echo imaging in the breast. J Magn Reson Imaging. 2014 Feb 1;39(2):332–8.
3. Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008 Jul;18(7):1307–18.
4. Kuhl CK, Klaschik S, Mielcarek P, Gieseke J, Wardelmann E, Schild HH. Do T2-weighted pulse sequences help with the differential diagnosis of enhancing lesions in dynamic breast MRI? J Magn Reson Imaging. 1999 Feb 1;9(2):187–96.
5. Hochman MG, Orel SG, Powell CM, Schnall MD, Reynolds CA, White LN. Fibroadenomas: MR imaging appearances with radiologic-histopathologic correlation. Radiology. 1997 Jul 1;204(1):123–9.
6. Brink WM, Gulani V, Webb AG. Clinical applications of dual-channel transmit MRI: A review: Clinical Applications of Dual-Channel Transmit MRI. J Magn Reson Imaging. 2015 Oct;42(4):855–69.
7. Winkler SA, Rutt BK. Practical methods for improving B1+ homogeneity in 3 tesla breast imaging. J Magn Reson Imaging. 2015 Apr 1;41(4):992–9.
8. Hsu Y-C, Littin S, Chu Y-H, Lin F-H, Zaitsev M. Uniform Flip Angle 3D Tailored Excitation for MR Breast Imaging at 3T. In: Proc Intl Soc Mag Reson Med [Internet]. Honolulu, HI, USA; 2017 [cited 2017 Jul 31]. p. 4927. Available from: http://indexsmart.mirasmart.com/ISMRM2017/PDFfiles/4927.html
9. Amadon A, Cloos MA. Method and apparatus for compensating for B1 inhomogeneity in magnetic resonance imaging by nonselective tailored RF pulses. WO2011/128847A1, 2011.
10. Cloos MA, Boulant N, Luong M, Ferrand G, Giacomini E, Le Bihan D, et al. kT-points: Short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magn Reson Med. 2012 Jan;67(1):72–80.
11. Nistler J, Renz W. Method for controlling a magnetic resonance system [Internet]. US7847554 B2, 2010 [cited 2016 Nov 4]. Available from: http://www.google.ch/patents/US7847554
12. Busse RF, Hariharan H, Vu A, Brittain JH. Fast spin echo sequences with very long echo trains: Design of variable refocusing flip angle schedules and generation of clinicalT2 contrast. Magn Reson Med. 2006 May;55(5):1030–7.
13. Mugler JP. Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging. 2014 Apr 1;39(4):745–67.
14. Rakow-Penner R, Daniel B, Yu H, Sawyer-Glover A, Glover GH. Relaxation times of breast tissue at 1.5T and 3T measured using IDEAL. J Magn Reson Imaging. 2006 Jan 1;23(1):87–91.
15. Fautz HP, Vogel M, Gross P, Kerr A, Zhu Y. B1 mapping of coil arrays for parallel transmission. In: Proceedings of the 16th Annual Meeting of ISMRM, Toronto, Canada [Internet]. 2008 [cited 2015 Oct 29]. p. 1247. Available from: http://cds.ismrm.org/ismrm-2008/files/01247.pdf
16. Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984 Oct 1;153(1):189–94.
17. Gras V, Luong M, Amadon A, Boulant N. Joint design of kT-points trajectories and RF pulses under explicit SAR and power constraints in the large flip angle regime. J Magn Reson. 2015 Dec;261:181–9.
18. Hoyos-Idrobo A, Weiss P, Massire A, Amadon A, Boulant N. On variant strategies to solve the magnitude least squares optimization problem in parallel transmission pulse design and under strict SAR and power constraints. IEEE Trans Med Imaging. 2014;33(3):739–748.
19. Tomi-Tricot R, Gras V, Boulant N, Vignaud A, Amadon A. kT-Points Pulse Design at 7T: Optimization of Pulse and Sub-Pulse Durations. In: Magnetic Resonance Materials in Physics, Biology and Medicine [Internet]. Vienna, AT; 2016 [cited 2016 Oct 27]. p. 247–400. Available from: http://link.springer.com/10.1007/s10334-016-0570-3
20. Gras V, Mauconduit F, Vignaud A, Amadon A, Bihan DL, Stöcker T, et al. Universal pulse design of refocusing kT-point pulses using parallel transmission: application to three-dimensional T2-weighted sequences at 7T [Internet]. 2017 [cited 2017 Nov 5]. Available from: https://hal.archives-ouvertes.fr/hal-01567829/document
Figures