4338
In silico Platform for Evaluation of Constrained Reconstruction in DCE-MRI1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States, 3Carbone Cancer Center, University of Wisconsin-Madisonsin, Madison, WI, United States, 4Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 5Department of Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States, 6Department of Emergency Medicine, University of Wisconsin School of Medicine and Public Health, Madison, WI, United States
Synopsis
In this work, we show the value of a digital phantom to evaluate a dynamic reconstruction. We evaluated the fidelity of the reconstruction using SSIM measurements from simulations and three patients to support conclusions derived from the digital phantom. The highly configurative characteristics of the in-silico platform provide a tool for other researchers to test, evaluate and compare their own acquisition and reconstruction techniques.
Introduction
A significant gap exists in the ability to validate and compare the plethora of constrained reconstruction methodologies being applied to DCE-MRI of cancerous lesions. Considerable difficulty, time and expense is incurred identifying appropriate subjects with the desired range in morphological and temporal enhancement patterns while also identifying a gold standard for the actual spatial and temporal enhancement pattern of each subject. We integrate digital components to create spatially and temporally realistic breast lesions and merge them into realistic breast background tissue. The purpose of this work is to demonstrate utility of the in silico platform by evaluating an extended version of the dynamic reconstruction from our previously presented abbreviate DCE breast MRI protocol1.Methods
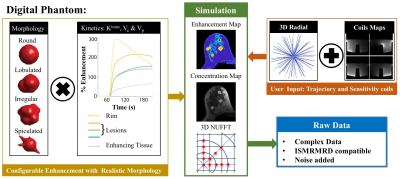
A digital breast phantom tool has been developed at our institution2-4 that creates variants of breast lesions with reasonable morphology and enhancement patterns derived from user-selected pharmacokinetic parameters, as shown in Figure 1. Raw data in the ISMRMRD format is generated to simulate data weighted by user-specified coil sensitivity maps, acquired along user-specified trajectories, and degraded by realistic noise.
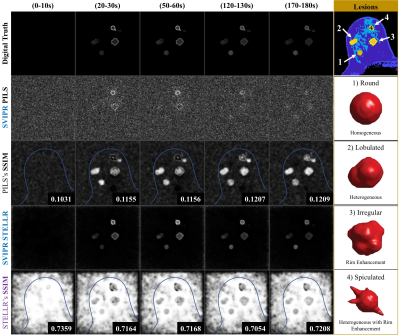
We generated raw data to simulate four lesion variants (Figure 3) merged into a realistic breast background being acquired by a 3D radial mask-subtracted (SVIPR) trajectory. To simplify analysis, a step-function transition was used in the two spatially heterogeneous lesions between the core and ring-enhancing region.
We evaluated the utility of the digital phantom by evaluating:
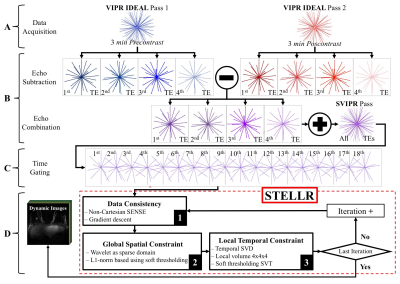
- A new reconstruction methodology we developed that dovetails with 3D radial acquisitions, Spatial CS with Temporal Local Low-Rank assistances—STELLR (Figure 2).
- A simple Parallel Imaging reconstruction (PILS)5-based strategy.
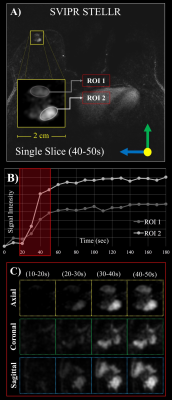
STELLR exploits sparsity created by pre-contrast mask-subtraction, a spatial constraint and local low-rank constraint in the temporal dimension to produce bilateral breast images with 10 second frame rates and 0.83 mm resolution. In vivo STELLR studies were used to assure that the stochastic noise level in the digital simulation was representative of actual scans. We benchmarked the temporal and spatial fidelity of both methods by computing the global value and similarity image of the Structural Similarity Index Measurements (SSIM) 6,7.
In-vivo comparison: Three patients consented to a research DCE MRI, IRB-approved, HIPAA compliant study using a 16-channel breast coil (Discovery 750 3T, GE Healthcare) over a 32 cm FOV. SVIPR, a T1-weighted 3D radial trajectory8 with four unique echoes was used to acquire one pre- and one post-contrast phase, each 3 minutes long (Fig. 2a).
Results
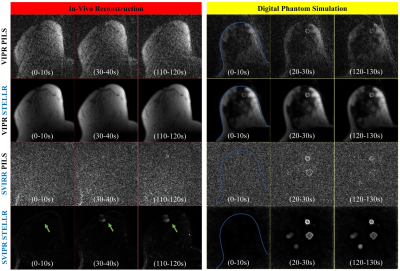
PILS reconstruction failed to provide a proper visualization of the expected enhancement pattern. On the contrary, the dynamic non-linear STELLR approach was able to overcome the significant data undersampling (45x acceleration). The STELLR reconstruction provided detailed visualization of homogeneous and heterogeneous lesions morphology. The value of the SSIM similarity image is demonstrated in the bottom row of Figure 3, where the darkened regions in the speculated lesion show the difficulty in accurately depicting the rim enchainment in this challenging lesion. The digital phantom successfully mimicked in vivo performance, as shown in the comparison of in-vivo and in silico enhancement in Figure 4. STELLR relies on mask subtraction to increase sparsity and achieved maximum performance. Finally, in vivo reconstructions of a volunteer in Figure 5 showing morphologic details of a known breast cancer, including spiculated margins and an irregular shape corroborate the digital phantom’s predicted performance.Discussion
Due to the lack of a gold standard for high temporal resolution dynamic reconstructions, definitive validation of temporal and spatial performance claims is difficult. Some groups have shown the value of physical phantoms9,10 and a dynamic digital phantom11 to accelerate and ensure validation of new MR techniques using realistic digital imaging features and kinetic behavior. The advanced digital phantom described here could evaluate new acquisition and reconstruction performance for depicting specific in vivo physiology and/or heterogeneous treatment response prior to undergoing the expense and efforts of clinical trials. The highly configurative characteristics of the digital phantom and its ISMRM compatible formats will allow us and others to compare reconstruction methods within and across institutions.Conclusion
We demonstrated the value of a digital phantom to evaluate the performance of the SVIPR STELLR approach. The in-silico simulation allowed for quantitative measurements. The in-silico platform can be configured or adapted to other scenarios to satisfy researcher’s necessities beyond clinical evaluation of new DCE-MRI methods.Acknowledgements
Research supported by NIH R25 GM083252, K24 DK102595, T32CA009206, and F31CA217160, RSNA Research & Education Foundation, the Department of Radiology R & D Fund at the University of Wisconsin, Wisconsin Women's Health Foundation, the Science and Medicine Graduate Research Scholars and GE Healthcare.References
- Jimenez JE, Johnson KM, Henze Bancroft LC, Hernando D, Strigel MR, Reeder SB, Block WF. 10 Second Temporal Resolution of Early Enhancement Visualization: Framework for Fast Breast MRI Screening. 2017; Honolulu, USA.
- Henze LC, Moran CJ, Smith MR, Kelcz F, Samsonov A, Fain SB, Block WF. Deterministic Comparisons of Nonlinear Acceleration Methods Using a Realistic Digital Phantom. 2010; Stockholm Sweden.
- Henze LC, Moran CJ, Smith MR, Kelcz F, Samsonov A, Fain SB, Block WF. Digital Breast Phantom for Evaluating Dynamic Accelerated Imaging Methods. 2010; Stockholm Sweden.
- Henze Bancroft LC, Wu D, Bosca RJ, Morrison CK, Block WF, Korosec FR, Strigel RM. The Impact of Accelerated Imaging on Breast DCE MRI: Analysis of a 3D Radial Reconstruction using a Digital Breast Phantom. 2015; San Francisco, CA.
- Griswold MA, Jakob PM, Nittka M, Goldfarb JW, Haase A. Partially parallel imaging with localized sensitivities (PILS). Magn Reson Med 2000;44(4):602-9.
- Zhou W, Bovik AC, Sheikh HR, Simoncelli EP. Image quality assessment: from error visibility to structural similarity. IEEE Transactions on Image Processing 2004;13(4):600-612.
- Kumar B, Kumar SB, Kumar C. Development of improved SSIM quality index for compressed medical images. 2013 9-11 Dec. 2013. p 251-255.
- Moran CJ, Brodsky EK, Bancroft LH, Reeder SB, Yu H, Kijowski R, Engel D, Block WF. High-resolution 3D radial bSSFP with IDEAL. Magn Reson Med 2014;71(1):95-104.
- Ramsay E, Causer P, Hill K, Plewes D. Adaptive bilateral breast MRI using projection reconstruction time-resolved imaging of contrast kinetics. J Magn Reson Imaging 2006;24(3):617-24.
- Saranathan M, Rettmann D, Hargreaves BA, Lipson J, Daniel BL. High Spatio-temporal Resoltuion breast dynamic contrast enhanced MRI at 3T. 2012 Melbourne, Australia. p 456.
- Le Y, Kipfer H, Majidi S, Holz S, Dale B, Geppert C, Kroeker R, Lin C. Application of Time-Resolved Angiography With Stochastic Trajectories (TWIST)-Dixon in Dynamic Contrast-Enhanced (DCE) Breast MRI. Journal of Magnetic Resonance Imaging 2013;38(5):1033-1042.
- Morris EA, Comstock CE, Lee CH, Lehman CD, Ikeda DM, Newstead GM, Tozaki M, Hylton N, Helbich TH, Kuhl C and others. ACR BIRADS Magnetic Resonance Imaging. ACR BIRADS Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013.
Figures