4331
The Effect of Intravenous Administration of a Gadolinium-Based Contrast Agent on Breast Diffusion Tensor Imaging: Qualitative and Quantitative Evaluation.1Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada, 2Marvelle Koffler Breast Centre, Sinai Health System, Toronto, ON, Canada, 3Toronto Joint Department of Medical Imaging, University of Toronto, Toronto, ON, Canada, 4Weizmann Institute of Science, Rehovot, Israel, 5Toronto Joint Department of Medical Imaging, University Health Network, Toronto, ON, Canada
Synopsis
We have investigated whether the values of the diffusion tensor imaging (DTI) parameters of breast normal tissue, as well as of benign and cancer lesions are affected by gadolinium-based contrast administration. Changes in the DTI parameters and consequently in DTI-based lesion size were evaluated pre and post dynamic contrast enhanced (DCE) MRI. Results indicated that scanning with DTI post DCE did not impact the diffusion parameters in breast normal tissue and benign lesions and the lesions’ size but revealed a significant reduction of the diffusion coefficients in breast cancers, suggesting potential improvement of DTI diagnostic specificity post-contrast.
Purpose:
To quantify the changes in the diffusion tensor parameters pre and post administration of a gadolinium-based contrast agent (CA) and investigate the influence of the CA on the efficiency of these parameters to diagnose breast cancer.Introduction:
In recent years diffusion-weighted imaging (DWI) is increasingly performed in breast MRI as an adjunct tool to dynamic contrast-enhanced (DCE) MRI1-3. MRI diffusion measurements are based on intrinsic contrast, hence, are completely noninvasive. DWI measures an averaged apparent diffusion coefficient (ADC). Diffusion tensor imaging (DTI) extends the averaged information of DWI to symmetric tensor metrics allowing parametric mapping of directional diffusion coefficients (DDCs), anisotropy indices (AIs) and mean diffusivity (MD), as well as tracking architectural features. Very little is known about the effects of MRI CAs on the water diffusion parameters of the normal and malignant breast tissue, and therefore, in most studies, DWI or DTI protocols are applied prior to a DCE protocol. However, as DCE is the standard protocol, it is important for optimization and standardization of breast MRI to determine the effect of CAs on the diagnostic efficiency of the diffusion parameters.Methods:
Twenty six consecutive women (BIRADS 0, 4, 5 or 6 on conventional breast imaging) underwent diagnostic MRI. Images were acquired on a 3T scanner (Skyra-Fit, Siemens) with a 16-channels breast coil. The MRI protocol included axial T1 and T2-weighted images without and with fat suppression, DTI with a spin-echo EPI sequence (1.875x1.875x2.4mm3 resolution, TE/TR= 86ms/12600ms, b-values 0/700 s/mm2 and 30 diffusion-gradient directions), and a DCE protocol (TE/TR/flip-angle=1.72ms/3.86ms/18°, 0.72x0.72x1.2mm3 or 1.1x0.8x1.1mm3 resolution) 1 pre- and 5 post- i.v. Gadobutrol administration (0.1 mmol/kg at 2.0 ml/s with 20ml saline flush). The DTI protocol was acquired twice: pre and immediately after DCE (~6 min after the i.v. CA administration). Slice thickness in the T1-w, T2-w and DTI protocols was identical.
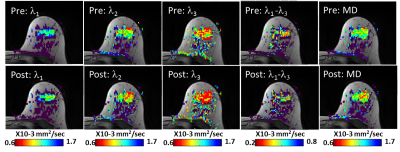
DTI datasets were analyzed using a proprietary software, yielding three eigenvectors and their corresponding eigenvalues (termed DDCs), λ1, λ2, λ3 their MD and maximal AI, λ1-λ34. A trained radiologist reviewed the DTI parametric images and the clinical information. The identified lesions on DCE were correlated to the T1-w/T2-w images, and to the λ1 maps, and then ROI of the lesion boundary was delineated on each λ1 map, using a threshold ≤1.7x10-3 mm2/s. Similarly, normal breast tissue was delineated on the ipsilateral and contralateral breast tissue, where the region of largest amount of fibroglandular tissue, preferentially in the upper outer quadrant was elected. Lesions diameter was measured on the λ1 maps.
Median values of the DTI parameters were reported. Wilcoxon paired two tailed signed-rank test was applied to compare diffusion parameters and lesion size for pre and post-contrast measurements. P<0.05 was considered statistically significant.
Results:
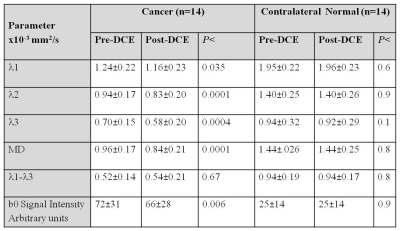
Of the 26 women, 15 had biopsy proven malignant lesions (13 IDC, 2 DCIS) and 11 had benign lesions (n=5) or normal findings (n=6). Median cancer size on pre-contrast DTI and post-contrast DTI was similar (p=0.35): 15.3 mm (range:3.3-72.3 mm) and 17.3mm (range: 3.9-71.0 mm), respectively. One DCIS lesion measuring 3.3 mm on pre-contrast DTI was excluded due to it's small size and potential miss localization. In normal fibroglandular tissue ROIs of the entire cohort of normal fibroglandular tissue in either breast and in the benign lesions the DDCs and maximal AI obtained post-contrast were not significantly different from those obtained pre-contrast (p>0.05). In the malignant lesions there was a significant reduction in the DDCs and MD, while there was no change in the maximal AI (Figure 1). Signal intensity on the b=0 images of the cancers showed a significant reduction post-contrast (Table 1), presumably due to the gradual T2 shortening by the remaining CA. However, no significant change was found in the b0 signal intensity post-contrast of benign/normal tissue, as CA influx to these regions was low.Discussion:
This pilot study indicated that scanning with DTI post DCE did not impact the diffusion parameters in normal breast tissue or benign lesions but revealed a significant reduction in the diffusion coefficients of cancers. Consequently, the conspicuity of the cancers increased leading to a higher degree of confidence by the user’s visual assessment, suggesting that DTI can be used for lesion detection post-contrast and may even improve diagnosis. These findings are in agreement with previous DWI studies measuring ADC3,5,6 that demonstrated a slightly better lesion discrimination post-contrast. Additional clinical studies comparing the effect of various CAs and the DTI timing need to be performed in order to standardize breast MRI protocol and validate that post-contrast DTI may improve the specificity of DTI.Acknowledgements
We thank to Dr Pavel Crystal "ז״ל." (deceased in 2016) for his full support of this study as the Divisional Head in Breast Imaging. AM Scaranelo receives research support via APT Program (TJDMI Canada). Dr. E. Furman-Haran holds the Calin and Elaine Rovinescu Research Fellow Chair for Brain Research. Prof. H. Degani holds the Fred and Andrea Fallek Chair for Breast Cancer Research.References
1. Chen X, Li WL, Zhang YL, et al. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer, 2010 Dec 29;10:693. doi: 10.1186/1471-2407-10-693.
2. Partridge SC, Nissan N, Rahbar H, et al. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J. Magn. Reson. Imaging, 2017 Feb;45(2):337-355. doi: 10.1002/jmri.25479.
3. Dorrius MD, Dijkstra H, Oudkerk M, et al. Effect of b value and pre-admission of contrast on diagnostic accuracy of 1.5-T breast DWI: a systematic review and meta-analysis. Eur Radiol, 2014 Nov;24(11):2835-2847. doi: 10.1007/s00330-014-3338-z.
4. Eyal E, Shapiro-Feinberg M, Furman-Haran E, et al. Parametric diffusion tensor imaging of the breast Invest. Radiol, 2012 May;47(5):284-291. doi: 10.1097/RLI.0b013e3182438e5d.
5. Yuen S, Yamada K, Goto M, et al. Microperfusion-induced elevation of ADC is suppressed after contrast in breast carcinoma. J. Magn. Reson. Imaging, 2009 May;29(5):1080-4. doi: 10.1002/jmri.21743.
6. Janka R, Hammon M, Geppert C, et al. Diffusion-weighted MR imaging of benign and malignant breast lesions before and after contrast enhancement. Rofo, 2014 Feb;186(2):130-5. doi: 10.1055/s-003-1350298.
Figures