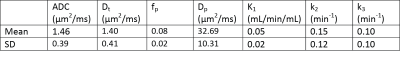4327
Diffusion-weighted Intravoxel incoherent motion (IVIM) MRI and dynamic 18F-FDG-PET imaging in breast cancer patients via simultaneous PET/MR1New York University School of Medicine, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, NYU Langone Medical Center, New York, NY, United States, 3Center for Advanced Imaging and Innovation (CAI2R), NYU Langone Medical Center, New York, NY, United States, 4Department of Radiology, NYU Langone Medical Center, New York, NY, United States, 5Department of Internal Medicine, NYU Langone Medical Center, New York, NY, United States, 6Department of Surgery, NYU Langone Medical Center, New York, NY, United States, 7Imaging and Therapy Division, Siemens AG, Healthcare Sector, Erlangen, Germany
Synopsis
Aggressive breast tumors possess heterogeneity that impacts successful diagnosis and treatment. Mapping this complexity with imaging biomarkers of different biologic specificity supports patient-specific management. We compare biomarkers from diffusion-weighted MRI (intravoxel incoherent motion (IVIM)) and 18F-fluorodeoxyglucose (FDG) PET (dynamic pharmacokinetic modeling) in 10 breast cancer patients in a simultaneous PET/MR system. Voxelwise correlations were performed to study intralesion relationships between biomarkers. Intralesion correlations were observed, such as between PET plasma transfer rate K1 and tissue diffusivity Dt, that also showed potential diagnostic value in tumor classification. This feasibility study establishes a workflow that enables more detailed investigation in larger cohorts.
Introduction
The apparent diffusion coefficient (ADC) from diffusion weighted imaging (DWI) is known to differentiate between malignant and benign breast cancers.1,2 The DWI technique intravoxel incoherent motion (IVIM) further characterizes water motion by differentiating between tissue diffusion Dt and pseudo-diffusion/vascular flow Dp.3 Relationships have been demonstrated between IVIM parameters and breast cancer malignancy6-9 as well as molecular biomarkers.8,10,11 Positron emission tomography (PET), utilizing the radiopharmaceutical 18F-fluorodeoxyglucose (18F-FDG), is used clinically for the detection of metastases in cancer, including those of the breast.12,13 Previous work has assessed the relationship between diffusion metrics and standard uptake value (SUV) in breast14-16 and other cancers.17,18 However, analogously to ADC in diffusion MRI, SUV involves multiple factors (cell proliferation, vascularity, phosphorylation) that can limit its specificity. Combined FDG/IVIM metrics have the potential to provide complementary information which could improve monitoring and treatment. Therefore, we analyzed the intermetric relationship between diffusion metrics and dynamic PET pharmacokinetic parameters in a pilot cohort of patients with primary breast cancer lesions in a simultaneous PET/MR system.Methods
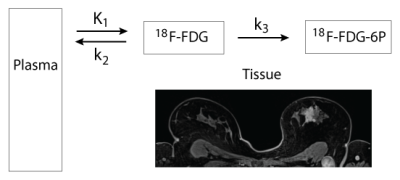
10 female patients (average age 54.6 ± 12.7) who gave written informed consent were imaged in this IRB-approved and HIPAA-compliant study. All patients had invasive ductal carcinoma confirmed by histopathology (2 with distant metastases, 4 with nodal metastases only, 4 with no metastases; ER 7+/3-; PR 6+/4-; Her2 3+/7-; Ki67 4+/6- using threshold 30%19). Images were acquired on an integrated 3T PET/MR Biograph mMR (Siemens Healthcare, Erlangen, Germany) with 18F-FDG injection performed 1 minute after acquisition start. DWI (TR/TE = 7300/94 ms; matrix 192 x 192; 20 slices; slice thickness 4 mm; 3 directions) were acquired at 7 b-values (0, 30, 70, 100, 200, 500, 800 s/mm2) with a prototype twice-refocused spin echo planar imaging (EPI) sequence with spectral attenuated inversion recovery, reversed slice gradient fat suppression, and eddy current distortion correction. Gadolinium contrast was injected prior to acquisition of T1 and iterative Golden-angle RAdial Sparse Parallel MRI (iGRASP)20 images. Dynamic PET images were reconstructed on vendor software from a 45-minute acquisition, incorporating a Dixon-based attenuation map and using Maximum Likelihood reconstruction of Attenuation and Activity (MLAA) algorithm.21 Retrospective registration was performed using in-house software (FireVoxel, wp.nyu.edu/firevoxel). All DWI were registered using a mutual information criterion restricted to a 3D lesion mask segmented on the iGRASP volume. PET images were aligned to iGRASP images using DICOM image position information. ADC and IVIM parameters (Dt, fp, Dp) were computed with custom code (IgorPro, Wavemetrics Inc., Portland, OR, USA). Raw PET data was converted to SUV using 18F-FDG dose and half-life, time elapsed since injection, instrumental calibration factors, and body mass. Pharmacokinetic parameters K1 (mL/min/mL), k2 (min-1), and k3 (min-1) were computed in Firevoxel using a 2-tissue irreversible model (Figure 1), with ascending aorta ROIs drawn on PET images for the arterial input function.22 Pearson correlation coefficients were calculated for voxelwise comparison of PET metrics and diffusion metrics. Cross-metric correlations were compared between patient groups defined by (a) metastatic status and (b) prognostic factors (ER/PR/Her2/Ki-67) with Student’s t-tests (IBM SPSS Statistics, Armonk, NY, USA).Results
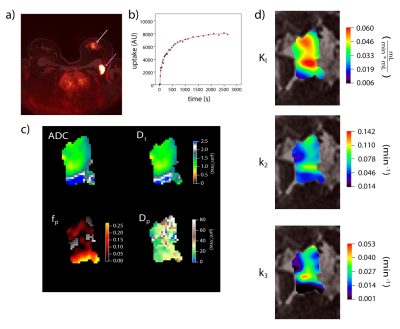
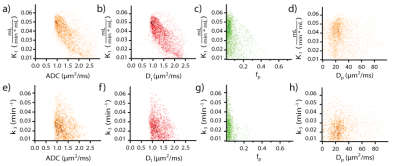
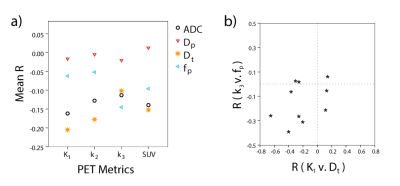
Mean lesion values of imaging biomarkers were consistent with known value ranges (Table 1). Figure 2 shows example lesion findings in a breast cancer patient with invasive ductal carcinoma (IDC) of the left breast. iGRASP/PET fusion images show lesion localization, from which dynamic PET uptake was recorded following injection. Parametric maps from diffusion imaging (ADC, Dt, fp, Dp) and dynamic PET modeling (K1, k2, and k3) illustrate lesion heterogeneity; cross metric correlations between K1 and diffusion metrics (Figure 3) reveal relationships, e.g. high K1 occurring with low ADC/Dt. At the group level, notable intralesion correlations were found between K1 and ADC/Dt, and secondarily between k3 and fp (Figure 4). Several correlation types showed significant differentiation of clinical factors: Ki-67 status (K1vDt, p=0.033; k2vDt, p=0.006; k2vADC, p=0.030); ER status (k2vADC, p=0.004; k3vADC, p=0.013; k3vDt, p=0.012); PR status (k2vADC, p=0.021); metastatic status (K1vDp, p=0.040).Discussion
This study shows the feasibility of collecting and correlating metrics from diffusion-weighted MRI and dynamic 18FDG-PET in breast cancer patients using a simultaneous PET/MR system. In addition to collecting average biomarkers, the workflow generates patient-specific markers of tumor heterogeneity via intralesional correlations. Some correlations found between the IVIM and dynamic PET parameters are stronger than those between the first order ADC and SUV parameters, supporting the value of detailed signal modeling. Furthermore, these correlations may have diagnostic value, as suggested by initial clinical differentiation of tumor types in this pilot cohort. Further recruitment and analysis may enable more detailed investigation of this diagnostic potential and its impact on individualized patient management.Acknowledgements
We thank Thorsten Feiweier and Berthold Kiefer (Siemens Healthineers) for continued support of the prototype diffusion MRI sequence employed in this study.References
1. Inoue K, Kozawa E, Mizukoshi W, et al. Usefulness of diffusion-weighted imaging of breast tumors: quantitative and visual assessment. Japanese journal of radiology. 2011;29(6):429-436.
2. Rubesova E, Grell AS, De Maertelaer V, Metens T, Chao SL, Lemort M. Quantitative diffusion imaging in breast cancer: a clinical prospective study. Journal of magnetic resonance imaging : JMRI. 2006;24(2):319-324.
3. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401-407.
4. Iima M, Le Bihan D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology. 2016;278(1):13-32.
5. Sigmund EE, Cho GY, Kim S, et al. Intravoxel incoherent motion imaging of tumor microenvironment in locally advanced breast cancer. Magnetic resonance in medicine. 2011;65(5):1437-1447.
6. Bokacheva L, Kaplan JB, Giri DD, et al. Intravoxel incoherent motion diffusion-weighted MRI at 3.0 T differentiates malignant breast lesions from benign lesions and breast parenchyma. Journal of magnetic resonance imaging : JMRI. 2014;40(4):813-823.
7. Iima M, Yano K, Kataoka M, et al. Quantitative non-Gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: differentiation of malignant and benign breast lesions. Investigative radiology. 2015;50(4):205-211.
8. Cho GY, Moy L, Kim SG, et al. Evaluation of breast cancer using intravoxel incoherent motion (IVIM) histogram analysis: comparison with malignant status, histological subtype, and molecular prognostic factors. Eur Radiol. 2016;26(8):2547-2558.
9. Liu C, Liang C, Liu Z, Zhang S, Huang B. Intravoxel incoherent motion (IVIM) in evaluation of breast lesions: comparison with conventional DWI. Eur J Radiol. 2013;82(12):e782-789.
10. Iima M, Kataoka M, Kanao S, et al. Intravoxel Incoherent Motion and Quantitative Non-Gaussian Diffusion MR Imaging: Evaluation of the Diagnostic and Prognostic Value of Several Markers of Malignant and Benign Breast Lesions. Radiology. 2017:162853.
11. Kim YJ, Ko K, Kim DH, et al. Intravoxel Incoherent Motion Diffusion-weighted MR Imaging of Breast Cancer: Association with Histopathological Features and Subtypes. Br J Radiol. 2016:20160140.
12. Crippa F, Agresti R, Seregni E, et al. Prospective evaluation of fluorine-18-FDG PET in presurgical staging of the axilla in breast cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1998;39(1):4-8.
13. Minamimoto R, Senda M, Jinnouchi S, Terauchi T, Yoshida T, Inoue T. Detection of breast cancer in an FDG-PET cancer screening program: results of a nationwide Japanese survey. Clinical breast cancer. 2015;15(2):e139-146.
14. Baba S, Isoda T, Maruoka Y, et al. Diagnostic and Prognostic Value of Pretreatment SUV in F-18-FDG/PET in Breast Cancer: Comparison with Apparent Diffusion Coefficient from Diffusion-Weighted MR Imaging. Journal of Nuclear Medicine. 2014;55(5):736-742.
15. Byun BH, Noh WC, Lim I, et al. A new method for apparent diffusion coefficient measurement using sequential F-18-FDG PET and MRI: correlation with histological grade of invasive ductal carcinoma of the breast. Ann Nucl Med. 2013;27(8):720-728.
16. Ostenson J, Pujara AC, Mikheev A, et al. Voxelwise analysis of simultaneously acquired and spatially correlated 18 F-fluorodeoxyglucose (FDG)-PET and intravoxel incoherent motion metrics in breast cancer. Magnetic resonance in medicine. 2017;78(3):1147-1156.
17. Ho KC, Lin G, Wang JJ, Lai CH, Chang CJ, Yen TC. Correlation of apparent diffusion coefficients measured by 3T diffusion-weighted MRI and SUV from FDG PET/CT in primary cervical cancer. European journal of nuclear medicine and molecular imaging. 2009;36(2):200-208.
18. Schwenzer NF, Schmidt H, Gatidis S, et al. Measurement of Apparent Diffusion Coefficient With Simultaneous MR/Positron Emission Tomography in Patients With Peritoneal Carcinomatosis: Comparison With 18F-FDG-PET. Journal of Magnetic Resonance Imaging. 2014;40(5):1121-1128.
19. de Azambuja E, Cardoso F, de Castro G, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12 155 patients. Brit J Cancer. 2007;96(10):1504-1513.
20. Feng L, Grimm R, Block KT, et al. Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI. Magnetic resonance in medicine. 2014;72(3):707-717.
21. Nuyts J, Dupont P, Stroobants S, Benninck R, Mortelmans L, Suetens P. Simultaneous maximum a posteriori reconstruction of attenuation and activity distributions from emission sinograms. IEEE transactions on medical imaging. 1999;18(5):393-403.
22. Sokoloff L, Reivich M, Kennedy C, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. Journal of neurochemistry. 1977;28(5):897-916.
Figures