4326
The Added Utility of Diffusion Tensor Imaging for Differentiating Malignant and Benign Breast Lesions on 3T MRI: A Machine Learning Based Approach1Radiology, University of Washington School of Medicine, Seattle, WA, United States
Synopsis
Diffusion tensor imaging (DTI) may provide additional information on tissue characteristics over dynamic contrast enhanced (DCE) MRI, however there are conflicting results regarding its utility. Our study evaluated DCE and DTI features of histologically proven breast lesions on 3T MRI. Using a machine learning-based LASSO approach for multivariate regression and bootstrap-based internal validation, the model incorporating DCE and DTI parameters demonstrated significantly better performance in differentiating malignant and benign lesions compared to models using DCE or DTI parameters alone. These findings suggest that the addition of DTI sequences to DCE MRI may improve diagnostic performance.
Purpose
Although dynamic contrast enhanced (DCE) breast MRI has high sensitivity for breast cancer, the overlapping appearances of benign and malignant entities can produce false positives and unnecessary biopsies 1-3. Diffusion weighted imaging (DWI) has emerged as an adjunct to DCE MRI that can improve the detection and characterization of breast cancer 4, 5. Multiple prior studies have shown that breast cancers feature restricted diffusion on DWI with correspondingly low apparent diffusion coefficient (ADC) values compared to normal fibroglandular tissue 6. Diffusion tensor imaging (DTI) is an extension of conventional DWI that provides additional information on the direction and anisotropy of diffusion. Previous studies examining DTI parameters, such as fractional anisotropy (FA) and diffusion eigenvalues (λ1, λ2, λ3), have produced conflicting results regarding the added utility of DTI. While some studies have reported lower FA in benign lesions compared to malignant lesions, others have found no significant difference 6. The purpose of our study was to evaluate DCE and DTI features of histologically proven malignant and benign breast lesions on 3T MRI and to determine whether DTI can improve diagnostic performance.Materials and Methods
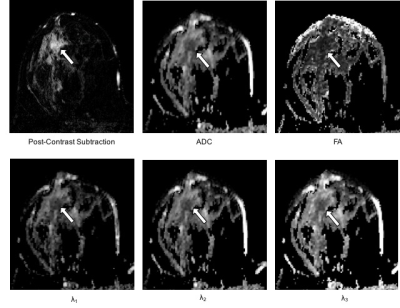
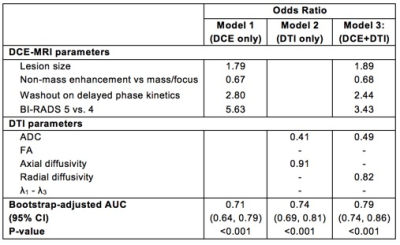
This IRB-approved prospective study included 199 women with MRI-detected BI-RADS 4 and 5 lesions who underwent core needle biopsy and/or surgical excision (October 2010 to December 2013). Breast MRIs included DCE and DTI sequences (b=0, 100, 800 s/mm2, 6 gradient directions). Four DCE parameters (lesion size, lesion type, presence of washout curve type, BI-RADS category) were recorded prospectively by interpreting radiologists, and five DTI parameters (ADC, FA, axial diffusivity [λ1], radial diffusivity [(λ2 + λ3)/2], and λ1 - λ3) were measured retrospectively by research scientists using in-house software developed in Image J (NIH, Bethesda, MD) blinded to final pathology (Figure 1). Univariate associations between imaging parameters and malignant/benign status were assessed using generalized estimating equations based logistic regression to account for any correlation between lesions from the same patient (odds ratios for continuous variables were scaled to show difference per 1-SD increase). Multivariate logistic regression models were developed using the least absolute shrinkage and selection operator (LASSO), which is a machine learning technique that simultaneously performs variable selection and parameter regularization to limit overfitting 7. Three separate models were made based on DCE parameters only, DTI parameters only, and the combination of both. The area under the receiver operating characteristic (ROC) curve (AUC) was used to measure model performance for discriminating between malignant and benign lesions. The bootstrap was used to adjust AUC estimates to account for training and testing using the same data set and to compare adjusted AUC values between models 8.Results
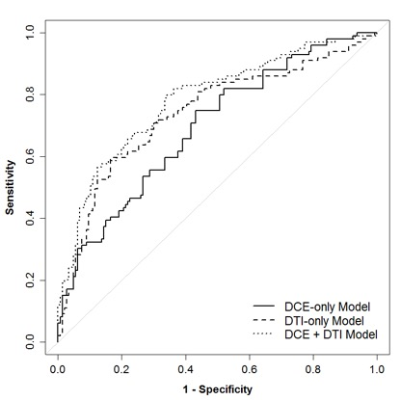
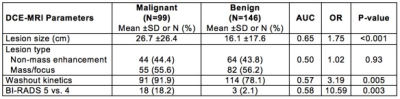
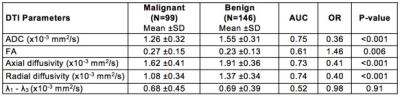
The study included 245 suspicious MRI-detected breast lesions in 199 patients (99 malignant, 146 benign). In univariate analysis, three DCE parameters showed significant differences between malignant and benign lesions: malignancies were larger in size (26.7 vs. 16.1 cm, p=< 0.001), more likely to demonstrate washout kinetics (91.9% vs. 78.1%, p= 0.005), and more likely to be characterized as BI-RADS 5 based on overall DCE MRI characteristics (18.2% vs. 2.1%, p=0.003; Table 1) than benign lesions. Four DTI parameters exhibited significant differences between malignant and benign lesions: malignancies had lower mean ADC (1.26 vs. 1.55, AUC=0.75, p=<0.001), higher FA (0.27 vs. 0.23, p=0.006), lower axial diffusivity (1.62 vs. 1.91, p<0.001), and lower radial diffusivity (1.08 vs. 1.37, p<0.001; Table 2) than benign lesions. Malignancies did not differ significantly from benign lesions by DCE lesion type (p=0.93) or DTI λ1 - λ3 (p=0.91). For the multivariate models, the LASSO selected all four DCE parameters for the DCE-only and combined models (Table 3). The LASSO selected ADC for the DTI-only (OR=0.41 per 1-SD increase) and combined models (OR=0.49) and also axial diffusivity (OR=0.91) and radial diffusivity (OR=0.82) for each model, respectively. Diagnostic performance of the DCE-only (bootstrap-adjusted AUC=0.71) and DTI-only models (adj. AUC=0.74) were not significantly different (ΔAUC=0.04, 95% CI: -0.05 to 0.13, p=0.43). The DTI-only model did not have significantly better performance than a univariate ADC-only model (ΔAUC=-0.01, 95% CI -0.01 to 0.02, p=0.52). Diagnostic performance of the combined DCE+DTI model (adj. AUC=0.79) was significantly better than the models based on DCE or DTI parameters alone (p<0.001 for both; Table 3; Figure 2).Conclusion
The multivariate LASSO model incorporating ADC and radial diffusivity, along with DCE parameters demonstrated the best diagnostic performance in differentiating malignant and benign lesions (AUC 0.79) compared to models using DCE or DTI alone (AUC 0.71 and 0.74 respectively). These findings suggest that the addition of DTI sequences to DCE breast MRI at 3T may improve the ability to distinguish between benign and malignant lesions.Acknowledgements
Funding for this study was provided by NIH grant R01CA151326References
1. Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244(2):381-8.
2. Elmore JG, Armstrong K, Lehman CD, Fletcher SW. Screening for breast cancer. JAMA. 2005;293(10):1245-56.
3. Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246(1):116-24.
4. Chen X, Li WL, Zhang YL, Wu Q, Guo YM, Bai ZL. Meta-analysis of quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesions. BMC Cancer. 2010;10:693.
5. Zhang L, Tang M, Min Z, Lu J, Lei X, Zhang X. Accuracy of combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging for breast cancer detection: a meta-analysis. Acta Radiol. 2016;57(6):651-60.
6. Partridge SC, Nissan N, Rahbar H, Kitsch AE, Sigmund EE. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J Magn Reson Imaging. 2017;45(2):337-55.
7. Tibshirani R. Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society, Series B. 1996;58(1):267-88.
8. Steyerberg EW, Harrell FE, Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774-81.
Figures