4319
Development of single-sided portable NMR methods for the sensing of mammographic density1School of Chemistry, Physics and Mechanical Engineering, Queensland University of Technology, Brisbane, Australia, 2School of Biomedical Sciences, Queensland University of Technology, Brisbane, Australia, 3Radiology, Princess Alexandra Hospital, Woolloongabba, Australia
Synopsis
Single-sided NMR was used to investigate the ability of spin-relaxation time constants to distinguish between regions of low and high mammographic density in human breast tissue. Measurements were performed on breast slices obtained from women undergoing breast reduction surgery or prophylactic mastectomy. T1 values in regions of high mammographic density were found to be significantly different to those measured in regions of low mammographic density. The findings suggest that portable NMR may be suitable for quantification of mammographic density in the breast tissue, presenting a promising and low-cost means of MD assessment in vivo without the use of ionising radiation.
Background
Mammographic density (MD) is a major independent risk factor for breast cancer. As such, it is a promising target for risk-modifying interventions. Mammographically dense breast tissue contains a large proportion of fibroglandular tissue, while low-density tissue is predominantly adipose. Traditionally MD is evaluated using X-ray mammography, where mammographically dense breast tissue appears radiopaque. However, the use of ionising radiation makes X-ray mammography unsuitable for high-frequency monitoring of MD. There is a significant interest in the development of an affordable and accurate method for quantifying MD that is free of ionising radiation, e.g. for the screening of cancer risk factors in younger women and for the monitoring of response to interventional cancer treatments.
Portable single-sided nuclear magnetic resonance (portable NMR) is a low-cost, low-maintenance, mobile technology that has been successfully used in industrial and manufacturing settings and is finding biomedical applications. The technology was developed in the late 1990s and has been available commercially for the last decade. Advantageous features of portable NMR include low cost, portability and low maintenance requirements. It enables one-dimensional depth profiling of a small region-of-interest, and therefore represents the middle ground between X-ray mammography (2D resolution only) and clinical MRI (fine 3D resolution but entails high installation and operational costs). It also offers many of the “traditional” quantitative MRI measurement modalities, including quantitative diffusion, T1 and T2 pulse sequences. This project aims to investigate the feasibility of distinguishing between high- and low-mammographic density breast tissue on the basis of non-invasive sensing using single-sided portable NMR.
Methods
We have used a commercially available portable-NMR instrument (NMR-Mouse) for all measurements. The samples measured included several sets of slices of human breast tissue from prophylactic mastectomies, smaller excised samples from both high- and low-MD regions, as well as artificial phantoms. Quantitative T2 (CPMG) measurements were performed on all the samples measured. A subset of samples was also investigated using quantitative T1 measurements (CPMG-detected saturation recovery). T1 data was analysed using least-squares fitting with a monoexponential recovery function. T2 data was analysed using inverse Laplace transform (ILT) of the CPMG decay curves with a manually optimised ILT regularisation parameter.Results and Discussion
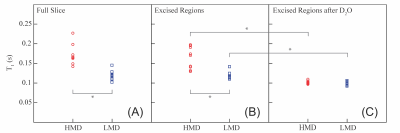
We have found that quantitative T1 measurements enable reliable differentiation between areas of high and low MD in breast tissue slices. Figure 1 shows a summary of the T1 results: part (A) shows the results of the measurements performed on the regions identified as HMD or LMD within the full slice; part (B) shows the results for the same regions but excised; and part (C) shows the results for the excised samples following H2O-D2O replacement (and therefore represents only the “fat” signal).
Both in the full slices and in excised samples, the mean T1 time constants in the HMD regions were longer than those in the LMD regions. In both scenarios, the difference between HMD and LMD regions was statistically significant (p < 0.001).
Upon the H2O-D2O replacement, both the HMD and LMD excised regions of the samples exhibited shorter T1 values with no statistically significant difference between the HMD and LMD regions. The spread of measured T1 values was reduced in the excised samples following H2O-D2O replacement. These results demonstrate that the adipose component of breast tissue exhibits a highly uniform T1 that is distinct from the water signal within the fibroglandular component. The results augur well for the relaxation-based quantification of the relative amounts of the adipose and fibroglandular components in breast tissue. This, in turn, is a promising proxy for MD.
Conclusion
This project is part of an ongoing investigation to determine whether portable NMR is capable of providing a safe, affordable and accurate methodology for the measurement of MD in vivo. The results are highly encouraging so far. Future work will include evaluation of different quantitative measurement modalities (especially diffusion and spin-relaxation) for non-invasive quantification of fibroglandular and adipose tissue in the breast.Acknowledgements
Funding from Princess Alexandra Research Foundation (ALH Breast Cancer Project Grant) and Translational Research Institute (SPORE Grant) is acknowledged. The Translational Research Institute is supported by a grant from the Australian Government. The authors are grateful to Dr Andrew Coy and Dr Robin Dykstra (Magritek Ltd) and A/Prof Petrik Galvosas (Victoria University Wellington, New Zealand) for the loan of PM5 NMR-MOUSE and invaluable discussions. MCT and TSA thank Dr R. Mark Wellard (QUT) for useful discussions concerning experimental design. The authors thank the women who gave permission for their breast tissue to be used for this study, Ms Gillian Jagger (Princess Alexandra Hospital) for the expert coordination of tissue accrual aspects of the project, and Ms Claire Davies for assistance with the accrual of the Mater Hospital specimen.