4306
Improved Efficacy of Cerebellar fMRI at 7T with Dielectric Pads Extending the Imaging Region of a Commercial Head Coil1Center for Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Bernard and Irene Schwartz Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York, NY, United States, 3Sackler Institute of Graduate Biomedical Sciences, New York University School of Medicine, New York, NY, United States
Synopsis
To improve cerebellar fMRI using a commercial head coil at 7T, we arranged High Permittivity Material (HPM) pads against the inferior, posterior region of the head. We first simulated the change in Specific energy Absorption Rate (SAR) with HPM and then examined the effects on experimental Signal to Noise Ratio (SNR), flip angle maps, and measured fMRI activation throughout the brain (including the cerebellum) during a finger-tapping task in eight subjects. Both SNR and fMRI BOLD contrast were improved in the cerebellum with no potential safety detriments.
PURPOSE:
Functional magnetic resonance imaging (fMRI) has facilitated non-invasive in vivo studies of brain function and connectivity. There is growing interest in studying the functional role of cerebro-cerebellar circuits (1-4), which requires robust sensitivity to blood oxygenation level dependent (BOLD) contrast throughout the whole brain. Ultra-high field MRI (>4 Tesla) is promising for fMRI, because of improved BOLD contrast (5). However, whole brain fMRI remains challenging, because commonly used head coils at 7 Tesla (T) have poor coverage in inferior regions of the brain and particularly in the cerebellum. Previous work has shown that appropriately placed HPM pads can extend the coil sensitivity into inferior regions of the brain and neck (6). In this work, we tested whether this extension in coil sensitivity can translate to an improvement in the detection of functional activation in the cerebellum for a finger tapping motor task in eight subjects.METHODS:
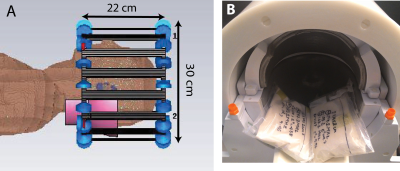
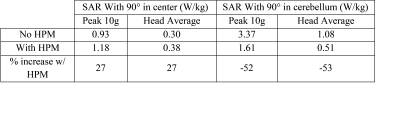
Simulations: Numerical simulations were performed to assess the Specific energy Absorption Rate (SAR) with and without HPM pads (εr=110 and σ=0.08 S/m) (Fig 1). A head-sized quadrature-driven birdcage coil, similar to the commercial transmit head coil used in the experiments (Fig. 1), was modeled using CST Microwave Studio 2015 (Computer Simulation Technology, Darmstadt, Germany), and loaded with a human model (“Duke,” Virtual Population, IT’IS Foundation, Zurich, Switzerland) (7) with a voxel resolution of 5x5x5mm3. Each port was tuned to 297.2 MHz, and matched to 50 Ohms in the absence of HPM. Simulations were performed with an accuracy of -30 dB to ensure convergence, and approximately 14 million mesh cells were used. The simulated 10 g local maximum SAR values were then scaled (8) to estimate SAR for the gradient-echo EPI sequence used in experiments. Experiments: Two identical HPM pads (12x14x2cm3) constructed from calcium titanate and deuterium oxide (9) were positioned inside a commercial head coil (Nova Medical 1Transmit/24Receive, Wilmington, MA) (Fig. 1). All studies involving human subjects were performed in accordance with our institution’s IRB. Eight healthy volunteers were enrolled in a prospective motor fMRI study, performed both with and without HPM. Volunteers were scanned on a whole-body 7 T scanner (Magnetom, Siemens Healthineers, Erlangen, Germany) using the commercial head coil. For each volunteer, the transmit reference voltage was set, with no HPM in the coil, to achieve a 90o flip angle at the center of the brain, using a turbo-FLASH based technique (10), and kept constant in the presence of HPM pads. To separate the receive-only coil contribution to SNR, SNR maps (11) were normalized by the sine of the flip angle maps (10), which were separately obtained. Functional activation maps were obtained using a gradient echo EPI sequence (101 measurements, slices=55, voxel size=2x2x2.5mm3, TR=3s, TE=23ms, bandwidth = 1930 Hz/pixel, flip angle = 15deg) for a right hand finger tapping task. fMRI data was analyzed using FEAT FSL (fMRI Expert Analysis Tool, version 6.0, FMRIB’s software Library, version 5.0.10) for each subject, followed by a group level analysis using paired t-test of acquisitions with and without HPM.RESULTS AND DISCUSSION:
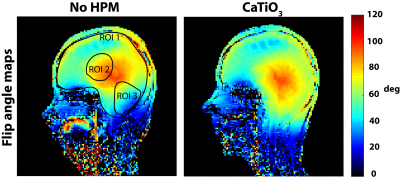
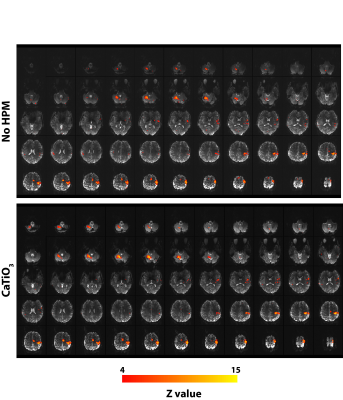
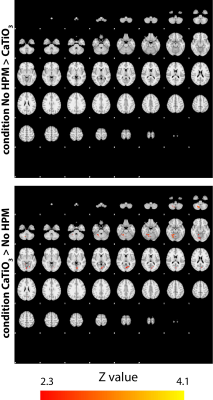
SAR values (Table) were well below the corresponding limits recommended by the FDA and IEC (12,13). Normalized experimental SNR maps demonstrated that the SNR improvement in the cerebellum (+26.79%) observed in the presence of HPM pads was primarily due to a larger achieved flip angle (+38.67%). The flip angle averaged across all subjects showed a mean improvement of 33% in the cerebellum and a decrease of 3.4% at the center, while the average flip angle in the whole brain remained approximately the same (Fig. 2). Functional MRI showed contralateral somatosensory motor cortical activation and activation of the superior and inferior axial slices of the ipsilateral cerebellar cortex (Fig. 3). In the presence of HPM pads a statistically significant improvement in the signal as well as the functional activation was observed for the inferior axial slices of the cerebellum (Fig. 3). This confirmed that an improvement in the flip angle (Fig. 2) can translate to an improvement in the detection of BOLD fMRI in the cerebellum. Group analysis results showed a statistically significant increase in activation in the cerebellar cortex with the HPM (Fig. 4), adjacent to the HPM pads (Fig. 1), and with no considerable loss elsewhere in the brain (Fig. 4).CONCLUSIONS:
HPM pads can improve cerebellar fMRI at 7T with a commonly used commercial head coil without compromising RF safety.Acknowledgements
This work was supported by the Center for Advanced Imaging Innovation and Research (www.cai2r.net), a NIBIB Biomedical Technology Resource Center (NIH P41 EB017183) and NIH R01 EB021277.References
1. Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage 2009;44(2):489-501.
2. Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. NeuroImage 2012;59(2):1560-1570.
3. Hui KKS, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N. The integrated response of the human cerebro-cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. NeuroImage 2005;27(3):479-496.
4. Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic Functional Connectivity As a Tool For Human Connectomics: Theory, Properties, and Optimization. Journal of Neurophysiology 2010;103(1):297-321.
5. Gati JS, Menon RS, Uǧurbil K, Rutt BK. Experimental determination of the BOLD field strength dependence in vessels and tissue. Magnetic Resonance in Medicine 1997;38(2):296-302.
6. Vaidya MV, Haemer G, Collins CM, Chen G, Carluccio G, Bruno M, Wiggins GC, Sodickson DK, Lattanzi R. Extending the Sensitivity of a Head Coil towards Simultaneous Head and Neck Imaging Using High Permittivity Materials at 7 T. In: Proceedings of the 24th Annual Meeting of the International Society for Magnetic Resonance in Medicine, Singapore, May 2016, p. 3544.
7. Andreas C, Wolfgang K, Eckhart GH, Katharina H, Marcel Z, Esra N, Wolfgang R, Rolf J, Werner B, Ji C, Berthold K, Peter S, Hans-Peter H, Jianxiang S, Michael O, Dominik S, Anthony K, Joshua WG, Niels K. The Virtual Family—development of surface-based anatomical models of two adults and two children for dosimetric simulations. Physics in medicine and biology 2010;55(2):N23.
8. Collins CM, Li S, Smith MB. SAR and B1 field distributions in a heterogeneous human head model within a birdcage coil. Magnetic Resonance in Medicine 1998;40(6):847-856.
9. Teeuwisse WM, Brink WM, Webb AG. Quantitative assessment of the effects of high-permittivity pads in 7 Tesla MRI of the brain. Magnetic Resonance in Medicine 2012;67(5):1285-1293.
10. Klose U. Mapping of the radio frequency magnetic field with a MR snapshot FLASH technique. Medical Physics 1992;19(4):1099-1104.
11. Kellman P, McVeigh ER. Image reconstruction in SNR units: a general method for SNR measurement. Magnetic Resonance in Medicine 2005;54(6):1439-1447.
12. Food and Drug Administration. Criteria for Significant Risk Investigations of Magnetic Resonance Diagnostic Devices - Guidance for Industry and Food and Drug Administration Staff. June 13, 2014.
13. International Electrotechnical Commission. Medical electrical equipment - Part 2-33: Particular requirements for the basic safety and essential performance of magnetic resonance equipment for medical diagnosis. IEC 60601-2-33 ed30 2010.
Figures