4303
Low-loss high-permittivity blocks improves the signal-to-noise ratio of surface coils at 3 Tesla1C.J. Gorter Center, Dept. Radiology, Leiden University Medical Center, Leiden, Netherlands
Synopsis
The performance of surface coils for proton imaging at 3 Tesla can be improved by placing low-loss high-permittivity blocks at the centre of the surface coil. The signal-to-noise ratio in a human calf of a coil with integrated dielectric block is 25% higher close to the coil and performs comparably well compared to a standard surface coil at depths exceeding 6 cm providing a simple and effective way of improving surface coil performance.
Introduction
Introduction Dielectric (or high-permittivity) materials have been used as a simple way to tailor B1+ fields in high-field MRI, either as a means to increase B1+ homogeneity1-3 or to produce intense local focussing of the B1+ field4, 5. Due to the local effect of the dielectric material on the B1+ field the dielectric materials are typically placed directly on the subject and underneath a tight fitting receive array. This study designs receive-only coil elements with the dielectric materials fully integrated in order to determine whether the receive sensitivity can be increased, as suggested by previous theoretical studies6. Electromagnetic simulations, phantom experiments and in-vivo imaging were performed to evaluate the effects on transmit and receive field efficiency of a coil with built-in dielectric compared to an identically sized coil with no dielectric.Method
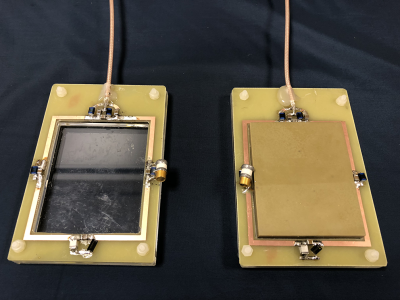
Two surface coils with inner dimensions of 73 × 60 mm2, conductor width of 3 mm, with 4 tuning capacitors and a balanced matching network were constructed. A rectangular dielectric block (70 × 57 × 10 mm3, εr = 660, σ = 0.01 S/m, fTE01δ = 267 MHz, mass = 210 gr) was placed in the centre of one of the surface coils (see figure 1) and both coils were subsequently tuned to the proton frequency at 3T. The S11 parameter of both coils was better than -20 dB when loaded with a tissue-mimicking phantom and placed under the human calf muscle. The signal-to-noise in the phantom was evaluated using a low flip angle single-slice gradient echo sequence (FoV = 220 x 400 mm2, In plane resolution 2.5 × 2.5 mm2, slice thickness = 10 mm, TR/TE = 3.69/1.73 ms, flip angle = 1°, scan duration = 4.84 seconds). A single-slice B1+ map was acquired using the DREAM sequence7 (FoV = 220 × 400 mm2, in plane resolution 2.5 × 2.5 mm2, slice thickness = 10 mm, TR/TESTE/TEFID = 4.63/1.6/2.30 ms, readout pulse flip angle = 10°, STEAM angle = 50°, number of signal averages = 32, scan duration = 93.4 s) to correct the SNR map for variations in signal intensity arising from variations in the flip angle due to B1+ variations. All data were acquired with the coils placed on a 35 x 25 x 12 cm3 phantom (εr = 40, σ = 0.7 S/m) on a clinical 3T MRI system (Philips Achieva, Philips Healthcare, Best, the Netherlands). In-vivo SNR data was acquired on healthy volunteers with the same scan sequences as described earlier.Results
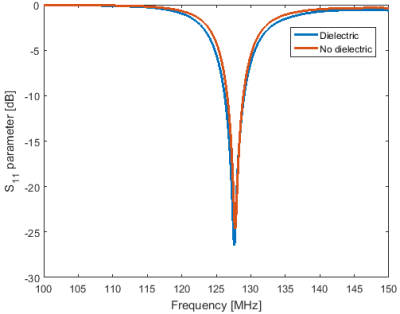
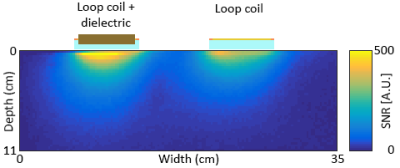
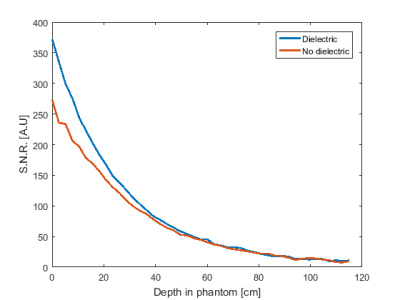
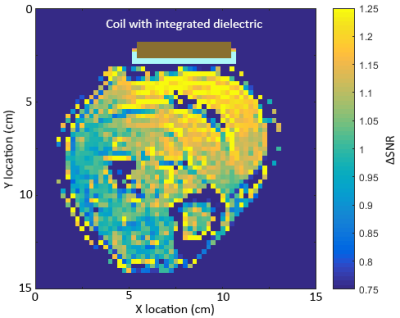
Figure 2 shows the S11 parameter plots of both the standard loop coil and the coil with integrated dielectric. A small drop in Q of the dielectric-integrated coil is observed compared to the standard surface coil, likely due to minor conductive losses inside the dielectric material. B1+ intensity in the phantom showed only minor changes when the dielectric block was added with a 5% increase a few centimetres from to surface of the block and no changes further away. Addition of the dielectric block did not significantly alter the shape of the dielectric integrated coil’s sensitivity profile compared to the regular surface coil. The observed SNR in a phantom near the surface of the coil, once normalised for variation in actual flip angle due to B1+ inhomogeneities, was 30% higher for the surface coil with integrated dielectric compared to the standard surface coil. SNR of the dielectric integrated coil exceeded the standard coil up to a depth of 3 cm after which both coils performed equally well. Figure 5 shows the change in SNR when using the surface coil with integrated dielectric compared to the reference surface coil on a human calf muscle. An SNR improvement of 25% is observed up to 3 cm away from the coil with performance of the dielectric integrated coil exceeding or matching the performance of the regular surface coil over the entire cross-section of the calf.Conclusion
Placement of a non-resonant low-loss high-permittivity dielectric block at the centre of a surface loop locally increases the SNR of the coil by up to 25% in-vivo while the penetration depth of the coil is maintained providing a relatively simple way to improve the performance of surface coils for proton imaging at 3T.Acknowledgements
This work was funded by the ERC, grant number 670629 and 737180 and by the NWO, grant number 13375.References
1. de Heer P, Brink WM, Kooij BJ, Webb AG. Increasing signal homogeneity and image quality in abdominal imaging at 3 T with very high permittivity materials. Magnet Reson Med 2012;68(4):1317-1324.
2. Teeuwisse WM, Brink WM, Webb AG. Quantitative assessment of the effects of high-permittivity pads in 7 Tesla MRI of the brain. Magnet Reson Med 2012;67(5):1285-1293.
3. Yang QX, Mao W, Wang J, Smith MB, Lei H, Zhang X, Ugurbil K, Chen W. Manipulation of image intensity distribution at 7.0 T: passive RF shimming and focusing with dielectric materials. J Magn Reson Imaging 2006;24(1):197-202.
4. Brink WM, van der Jagt AMA, Versluis MJ, Verbist BM, Webb AG. High Permittivity Dielectric Pads Improve High Spatial Resolution Magnetic Resonance Imaging of the Inner Ear at 7 T. Invest Radiol 2014;49(5):271-277.
5. Manoliu A, Spinner G, Wyss M, Ettlin DA, Nanz D, Kuhn FP, Gallo LM, Andreisek G. Magnetic Resonance Imaging of the Temporomandibular Joint at 7.0 T Using High-Permittivity Dielectric Pads: A Feasibility Study. Invest Radiol 2015;50(12):843-849.
6. Vaidya MV, Deniz CM, Collins CM, Sodickson DK, Lattanzi R. Manipulating transmit and receive sensitivities of radiofrequency surface coils using shielded and unshielded high-permittivity materials. MAGMA 2017.
7. Nehrke K, Bornert P. DREAM--a novel approach for robust, ultrafast, multislice B(1) mapping. Magnet Reson Med 2012;68(5):1517-1526.
Figures