4290
A 256-channel Cardiac Coil for accelerated Cardiac Imaging at 3 Tesla - Evaluation of a 32-channel Prototype1Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 2MR Coils B.V., Zaltbommel, Netherlands, 3Biomedical Engineering and Physics, Academic Medical Center, Amsterdam, Netherlands
Synopsis
There is an urgent need for highly accelerated cardiac imaging to facilitate shorter examination times and increased patient comfort. The purpose of this study was to investigate if a large number of small surface coils can increase the maximal obtainable acceleration factor, by maximizing SNR and minimizing the g-factor. We demonstrate the potential of a high-density cardiac receive array for faster cardiac imaging compared to current available MR coils. Measurements with a 32-channel cardiac prototype at 3T show a 4-fold increase in acquisition speed at similar SNR. At the cost of a small SNR-drop, 30-fold acceleration factors are possible.
Introduction
There is an urgent need for highly accelerated cardiac imaging to facilitate shorter examination duration and increased patient comfort [1]. However, the gain in speed by parallel imaging (PI) is limited by the signal-to-noise ratio (SNR), which decreases proportional to the coil geometry factor (g-factor) and the square root of the acceleration factor R [2]. It is suggested that a large number of small receiver coil elements, enclosing the region of interest, will maintain a low g-factor and therefore most optimal for accelerated imaging [3-6]. As such, a high-density cardiac array has the potential to substantially speed up image acquisition in patients undergoing cardiac MR examination.Purpose
The purpose of this study was to investigate if a high number of small surface coils can increase the maximal obtainable acceleration factor in PI, while maximizing SNR and minimizing the g-factor, with the aim of reducing scan time.Methods
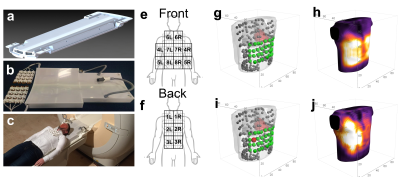
To evaluate the performance of a high-density, high-channel count cardiac coil array, a prototype surface coil array with 32 channels (see figure 1 a-c) was built. Measurements were performed on a Philips 3T Ingenia system with digital receivers (Philips, Best, Netherlands). Three subjects (large male, small male, female) were scanned with 4 different setups: The 1st setup consisted of a commercially available 28-channel array (16Ch anterior Torso Coil and the 12Ch posterior coil located in the patient table). The 2nd setup comprised of the 32-channel prototype coil in combination with the 12Ch posterior coil in the patient table. The 3rd and 4th setup consisted of 3 and 8 acquisitions with the 32Ch prototype, respectively, on different locations on the chest (see figure 1 e-f). These separately acquired datasets were co-registered to correct for subject motion, and merged into a virtual 96 and 256-channel cardiac array.
To evaluate the coil SNR and signal homogeneity a spin-echo sequence (TE = 1.29 ms, TR = 2.8 ms, FoV = 400x400mm2, 80 slices, slice thickness = 5mm, voxel size = 5x5x5mm3) was used, followed by noise scans that were obtained by switching off the gradients and the RF transmit power, while identical phasing and weigthing. To evaluate the 28 and 44 channel setups PI performance, a 3D b-TFE CINE was obtained (TE = 1.7 ms, TR = 4.4 ms, FOV = 384x384mm2, 180 slices, voxel size = 3x3x3mm3, Flip angle = 45 degrees, frames = 20).
Magnitude signals and noise images were reconstructed for all coil elements separately. The SNR of all channels combined was defined as $$$\frac{\sum_{n=1}^{N_{c}}(w_{n}S_{n})}{\sqrt{\sum_{n=1}^{N_{c}}(w_n^2}\sigma_n^2)}$$$, with $$$N_{c}$$$ the number elements, $$$S_{n}$$$ the signal of each element, $$$\sigma_n^2$$$ the noise standard deviation and $$$w_{n}$$$ the weighting of each element. Sensitivity profiles, used for element weighting, were recorded using reference scans. The g-factor and SNR-maps for all setups were calculated with SENSE accelerations in two directions [feet-head(FH), left-right (LR), anterior-posterior (AP)] for axial, coronal and sagittal slices through the heart.
Results
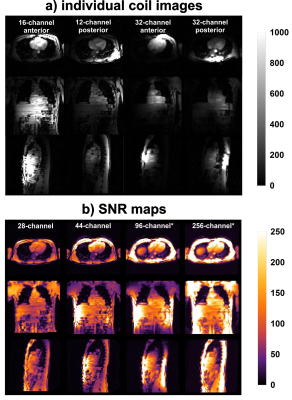
The signal from individual elements of the 16, 12 and 32-channel coils are shown in figure 2a. The penetration depth of each of the 32 elements is slightly lower, compared to the vendor setup. However, relative to the 28-channel setup, the overall SNR in the heart increased for all subjects (117.00±15.90%, 125.00±2.00%, and 178.00±12.80% for the 44, 96 and 256-channel coil arrays respectively, see figure 2b).
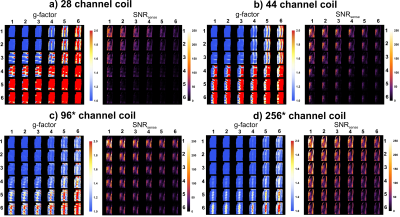
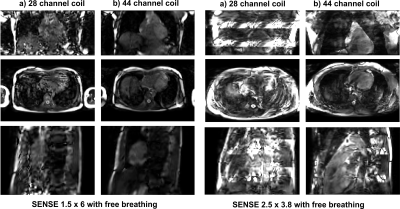
The g-factor maps and PI accelerated SNR maps for all four setups are shown in figure 3. The 44, 96, and 256-channel setups allowed for increasingly higher PI factors, up to 30, while maintaining a g-factor<1.5 (see table 1a). Importantly, using the 256-channel array a 16-fold acceleration is feasible with equal SNR, compared to 4-fold in the normal 28-channel setup (see table 1B). Figure 4 shows the ability to push PI acceleration even with the 44-channel setup beyond what is currently feasible with the default vendor setup.
Discussion
We demonstrated the potential of a high-density cardiac receiver array for faster cardiac imaging compared to current commercially available arrays. Measurements with the 32Ch Cardiac prototype demonstrate the potential for an approximately four-fold increase in acquisition speed at similar SNR. At the cost of a slight drop in SNR, acceleration factors of up to 30-fold seem feasible. With such acceleration factors in combination with 3D CINE imaging single breath hold whole heart acquisition with similar quality to current 2D acquisitions will be attainable and will potentially improve patient comfort and increase patient throughput in clinic.Conclusion
The 44-channel array demonstrated, how small high-density surface coils can dramatically improve PI accelerated scans. When expanding to 256-channels as simulated in this study, fast and comprehensive acquisition of cardiac MRI data would be feasible with up to 30-fold acceleration, while g-factors in the heart remain below 1.5.Acknowledgements
This project is financially supported by the Dutch Technology Foundation (TTW) and The Netherlands Heart Foundation - Grant Number 14741References
[1] M. Saeed, T. A. Van, R. Krug, S. W. Hetts and M. W. Wilson, "Cardiac MR imaging: current status and future direction," Cardiovascular Diagnosis & Therapy, vol. 5, no. 4, pp. 290-310, Aug 2015.
[2] K. P. Pruessmann, M. Weiger, M. B. Scheidegger and P. Boesiger, "SENSE: sensitivity encoding for fast MRI," Magn. Reson. Med., pp. 952-962, 1999.
[3] M. Schuppert, K. F. Kreitner, S. Fischer, S. Wein, B. Keil, L. L. Wald and L. M. Schreiber, "Determination of the optimal number of coil elements: a semi-theoretical approach," in ISMRM, 2015.
[4] R. Etzel, L. Golestanirad, C. Mekkaoui, T. G. Reese, D. E. Sosnovik, A. H. Mahnken and B. Keil, "Receive Coil Array considerations for simultaneous Multislice Imaging in Cardiac MRI," in ISMRM, 2017.
[5] M. Schmitt, A. Potthast, D. E. Sosnovik, J. R. Polimeni, G. C. Wiggins, C. Triantafyllou and L. L. Wald., "A 128-Channel Receive-Only cardiac coil for highly accelerated Cardiac MRI at 3 Tesla," Magn. Reson. Med., vol. 6, pp. 1431-1439, Jun 2008.
[6] C. J. Hardy, R. O. Giaquinto, J. E. Piel, K. W. Rohling, L. Marinelli, D. J. Blezek, E. W. Fiveland, R. D. Darrow and T. K. Foo, "128-channel body MRI with a flexible high-density receiver-coil array," J. Magn. Reson. Imag., vol. 5, pp. 1219-25, Nov 2008.
Figures