4281
One for all: Ultra-wideband (279-500MHz) self-grounded bow-tie antenna for ultrahigh-field and thermal MR1Berlin Ultrahigh Field Facility (B.U.F.F.), Max-Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2Department of Signals and Systems, Chalmers University of Technology, Gothenburg, Sweden, 3Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max-Delbrück Center for Molecular Medicine, Berlin, Germany, 4MRI.TOOLS GmbH, Berlin, Germany
Synopsis
A compact self-grounded ultra-wideband bow-tie antenna is presented that operates in a frequency range from 279 MHz (19F at 7.0 T) up to 500 MHz (1H at 11.7 T). The antenna is smaller and lighter than previous designs making it an excellent candidate for high density multi-channel transmit/receive arrays. It can be driven directly at 50Ω eliminating the need for a tuning/matching circuit and associated component losses and costs. The ultra-wideband antenna was tested successfully in-vivo at 7.0 T for 19F and 1H imaging. Its design supports high average power applications such as targeted RF heating or thermal MR.
Purpose
Temperature is a physical parameter with diverse biological implications and intense clinical interest. UHF MR is a promising tool for diagnosis including the spectrum of diagnostic imaging, non-invasive temperature monitoring and thermal MR interventions1-2. Possible clinical applications include mild hyperthermia treatments3-4 or controlled release of drug/contrast agent loads from thermoresponsive (nano)carriers5-6. A localized thermoresponsive release of fluorine labeled nanocarriers imposes challenging requirements on the RF antennae setup. High peak powers for 19F and 1H MRI at 7.0T, high average powers for targeted RF heating and a small antenna size that affords high density transmit/receive arrays are needed. In addition the ability of using higher RF frequencies is desirable to reduce the hotspot dimensions at the target7-8. For all these reasons we developed an ultra-wideband (UWB) antenna that operates in a frequency range of 279-500MHz (19F@7.0T to 1H@11.7T) and supports all the aforementioned applications. This antenna can be driven without a tuning/matching circuit. The performance of the antenna is benchmarked against existing designs and its application for in-vivo 1H and 19F MRI at 7.0T is demonstrated.Methods
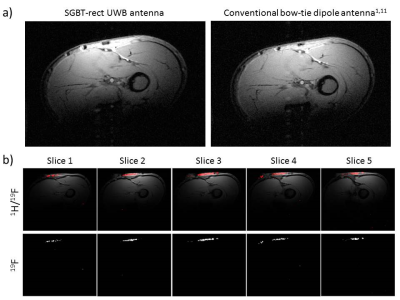
EMF simulations were performed in CST MWS 2015. A rectangular phantom (260x260x140)mm³ with frequency adjusted permittivity and conductivity of muscle tissue was used with three antenna types: (i) a bow-tie dipole antenna (BTA) in water (70x150x32)mm³ used for thermal MR1-2, (ii) an UWB self-grounded bow-tie antenna9-10 in water of conical shape (SGBT-cone) with diameter=80mm and height=26.5mm and (iii) an adapted version of the SGBT in water of rectangular shape (70x150x32)mm³ (SGBT-rect). The dimensions of the SGBT-rect (length=47mm,width=43.2mm) were adjusted to reach a minimum frequency of 279MHz (19F@7.0T). A layer of 10mm water was placed between phantom and antenna simulating a water bolus. The SGBT-rect antenna (Fig.1a) was constructed using 0.3mm thick copper. On the antenna wings a dielectric layer of FR-4 was used instead of PTFE10, avoiding fluorine that could contaminate 19F MR. A 50Ω coaxial cable feed was used without any tuning/matching circuit. The casing was 3D printed and filled with D20. S-parameters of the SGBT-rect were measured on a vector network analyzer (ZVT8,Rohde&Schwarz,Memmingen,Germany). 1H 2D gradient echo imaging was performed in a 7.0T MRI (Magnetom,Siemens Healthineers,Erlangen,Germany) on the forearm of a healthy volunteer using the SGBT-rect compared to a previously published BTA1,11. Combined 2D gradient echo 19F/1H imaging was performed on the same forearm after topical application of 101 mmol/L flufenamic acid – a 19F containing anti-inflammatory drug. 19F imaging protocols were adapted from a previous study12.Results
EMF simulation results show a good performance of B1+ and SAR at 300MHz and 500MHz of the suggested SGBT-rect (Fig.1b-d). B1+ and SAR at 300MHz is higher for SGBT-rect than for the BTA1 in surface regions (Fig.1d). This is expected since the SGBT-rect is shorter and closer to the phantom. At depth B1+ is comparable with both antenna designs. Although a conical design (SGBT-cone) shows good broadband behavior, a small airgap of 1mm introduces strong impedance changes (Fig.2b-c) leading to high reflection losses (Fig.2d). This is due to its strong self-resonance being slightly below 280MHz (Fig.2b-c). In comparison, the self-resonance of the SGBT-rect could be lowered substantially (Fig.2f-g), leading to S11<-10dB with a direct 50Ω feed over a frequency range of 272-487MHz (measured frequency range of 264-456MHz,Fig.3b). The coupling between two adjacent (10mm) antennas was max(S12)=-16dB at 276MHz (Fig.4). In-vivo imaging with the SGBT-rect antenna demonstrated comparable image quality for 1H at 7.0T versus the conventional BTA (Fig.5a). The SGBT-rect yielded slightly higher surface SNR which is in accordance with the EMF simulations (Fig.5a). 19F and 1H imaging on the forearm of a volunteer (Fig.5b) provided image quality comparable with that obtained from an 8-channel 1H/19F RF coil12.Discussion and Conclusion
A compact UWB antenna (SGBT-rect) is presented for UHF thermal MR applications ranging from 279MHz (19F@7T) to 500MHz (1H@11.7T). The antenna does not require a tuning/matching circuit, however a lower impedance further improves the antenna characteristics10 (Fig.2h). The SGBT-rect is less sensitive (vs. SGBT-cone) to introduced gaps between antenna dielectric and water bolus, allowing (i) to use D2O (no MR signal) as an intrinsic part of the antenna building block (ii) deionized H2O as a water bolus to improve EM-coupling to the body and reduce unwanted surface temperatures. SGBT-rect is smaller and at least 43% lighter than previous dipole designs using dielectrics1,13. This enables higher density transmit/receive arrays which is beneficial for targeted RF heating7-8 and accelerated MRI. One of the intended applications is the use of the antenna in a multi-channel array for localized thermo-activated drug release and monitoring with 19F/1H MR.Acknowledgements
This work was supported in part (LW, TN) by the Federal Ministry for Education and Research (BMBF, KMU innovativ Medizintechnik, 3-in-1 THERAHEAT, FKZ 13GW0102A and 13GW0102B).
The authors would like to thank Andre Kühne for helpful discussions.
References
1 Winter L, et al., PLoS One. 2013; 8:e61661; doi: 10.1371/journal.pone.0061661
2 Winter L, et al., Radiat Oncol. 2015; 10:201; doi: 10.1186/s13014-015-0510-9
3 Cihoric N, et al., Int J Hyperthermia. 2015; 31(6):609-14; doi: 10.3109/02656736.2015.1040471
4 Issels RD, et al., Lancet Oncol. 2010; 11(6):561-570; doi: 10.1016/S1470-2045(10)70071-1
5 Chilkoti A, et al., Adv Drug Delivery Rev. 2002; 54(5):613-630;
6 Langereis S, et al., J Am Chem Soc. 2009; 131(4):1380-1. doi: 10.1021/ja8087532
7 Guerin B, et al., Int J Hyperthermia. 2017; 11:1-14; doi: 10.1080/02656736.2017.1319077
8 Winter L and Niendorf T, MAGMA. 2016; 29(3):641-56; doi: 10.1007/s10334-016-0559-y
9 Yang J and Kishk A, IEEE Trans
Antennas Propaga. 2012; 60:1214–20; doi: 10.1109/TAP.2011.2180317
10 Takook P, et al., Int J Hyperthermia. 2017; doi: 10.1080/02656736.2016.1271911
11 Oezerdem, C, et al., Magn Reson Med. 2016; 75(6):2553-65; doi: 10.1002/mrm.25840
12 Ji Y, et al., NMR Biomed. 2015 Jun;28(6):726-37. doi: 10.1002/nbm.3300
13 Raaijmakers AJ, et al., Magn Reson Med. 2011; 66(5):1488-97; doi: 10.1002/mrm.22886
Figures