4280
Marmoset coil arrays for functional imaging of awake monkeys at 9.4T and 3T1Department of Radiology, A.A Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Department of Brain and Cognitive Sciences, MIT, Cambridge, MA, United States, 4Department of Bioengineering, MIT, Cambridge, MA, United States, 5Bruker Biospin, Billerica, MA, United States, 6McGovern Institute for Brain Research, MIT, Cambridge, MA, United States
Synopsis
High-field functional MRI of non-human primates provides elevated BOLD contrast in a well-established neuroscience model but requires an optimized coil design to boost the SNR, facilitate image acceleration while maintaining mechanical and electrical stability during the awake primate experiment. In this work we have constructed and tested 4-channel 9.4T and a 5-channel 3T receive-only array optimized for high-resolution whole-brain imaging of the anesthetized and awake marmosets.
Introduction
Functional MRI of awake non-human primates, especially in small primates like marmosets, present unique challenges due to increased demands on image encoding and SNR required for high resolution imaging, along with the need to mitigate body-motion induced susceptibility distortions. High field strengths and receive-coil arrays can both boost the sensitivity required for smaller voxel sizes, but require dedicated receive coil geometries for accelerated primate imaging studies and careful attention to the stresses of the primate and the high-field environment. Previous studies have illustrated the benefits of using custom built monkey coils for brain imaging [1]. In our work, we extend this philosophy of imaging marmosets also beyond 7T [2]. Here we present a 4-channel 9.4T and a 5-channel 3T coil, compatible with a head restraint system. The coil diameter was chosen as a trade-off between element count, depth penetration and whole-brain coverage. The coil array is embedded on a custom head restraint system for use across different bore sizes, providing mechanical stability while minimizing the distance between the array elements and the marmoset brain. The coverage and sensitivity of the array is demonstrated using in vivo images in marmosets.Methods
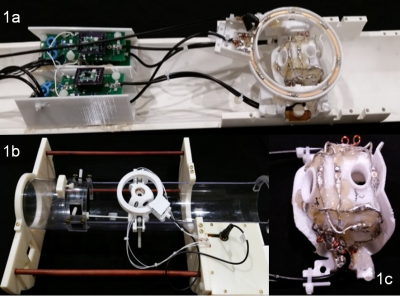
Fig. 1a shows the 4-channel 9.4T array with elements laid out on an ABS plastic former fabricated with a 3D printer, and positioned to surround the brain fully, while not obstructing the head-post system or the visual stimulation. Occipital and frontal port holes allow access to various brain areas to combine imaging with optogenetic manipulations and electrophysiological recording, as well as head-post implants, to address various experimental needs. Ports aligning ear canals accommodate custom designed ear bars. Fig. 1b shows the completed 5-channel 3T array. The array, transmit coil and preamps are mounted to the monkey cradle. The 4-channel 9.4T array (Fig. 1c) consists of four elements made of 16 AWG wire loops [3], 5 cm in diameter, with four equally-spaced capacitors. Preamp decoupling is achieved with a cable length of 25 cm, with the preamps placed some distance away to allow access and room for immobilization. This necessitated the use of lattice baluns at the drivepoint to each element to eliminate cable common-mode current issues. The lattice balun with a PIN diode provides matching and detunes each element during transmit. In addition to this, cable traps are placed behind the preamps. A 9 cm in diameter detunable single-loop coil provides excitation.
Data was acquired on an animal 9.4T scanner (Bruker Biospin, Billerica, MA) and the 3T scanner (Siemens Healthcare, Erlangen, Germany). Array noise covariance was estimated from thermal noise data acquired without RF excitation, and SNR maps were computed following the method of Kellman & McVeigh [4]. While comparing SNR maps between arrays at the 9.4T, the same transmit coil was used. We performed structural imaging in an adult marmoset, initially under anesthesia, using the 5-channel 3T array.
Results
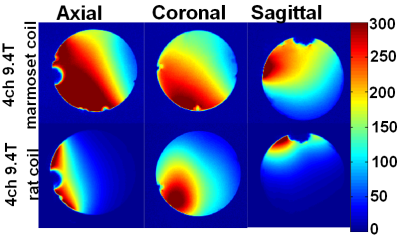
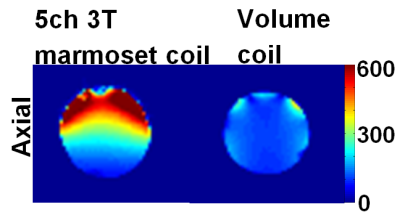
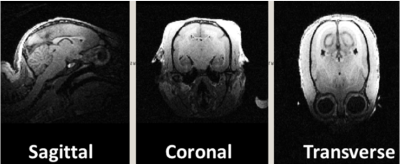
Each element (Fig. 1c) shows a Q unloaded-to-loaded ratio of ~260/100 with a spherical phantom 3.5 cm in diameter. Loaded S21 between neighboring elements ranges from ‑26 dB to ‑15.5 dB. S11 reflections showed the elements tuned and matched to 50 Ohms. Fig. 2 compares the SNR maps between the 4-channel 9.4T marmoset array and a 4-channel Bruker rat array applied to the 3.5cm phantom, showing about a significant SNR increase. Fig .3 compares the SNR maps between the 5-channel 3T marmoset array with a volume coil (diameter 63mm and length 51mm) showing the potential for SNR increases in many brain regions. The measured EPI time-course stability of the 4-channel 9.4T array coil was 1.3% for peak-to-peak and 0.2% for the 5-channel 3T array. It is to be noted the numbers are relative at each field strength but cannot be used to compare the coils at two different field strengths. Fig. 4 shows structural images of an adult marmoset brain at 350 um isotropic resolution with excellent temporal stability, no motion artifacts and high resolution throughout the brain. The entire assembly – head coil and cradle was easy to use for placing the animal, provided excellent stability across scans and uniform coverage of entire brain.Conclusions
The 9.4T 4-channel monkey coil and the 3T 5-channel monkey coil provides an increase in overall SNR compared to arrays not designed for marmoset imaging. The EPI stability was adequate, indicating the coil’s utility for functional brain imaging. This design meets the additional demands placed on the coil by the high field strength, the mechanical constraints of the monkey cradle and stimulus, and measurement apparatuses.Acknowledgements
Support from Simons Center for Social Brain and Poitras Foundation. Thanks to Jose Estrada, Gao Yang.References
[1] Mareyam et al. (2011) Proc ISMRM. [2] Papoti et al. (2017) MRM 78(1):387-98. [3] Wiggins et al. (2006) MRM 56:216-23. [4] Kellman et al. (2005) MRM, 54(6):1439-47.Figures