4265
Balanced multi-shot EPI for accelerated Cartesian MR Fingerprinting: An alternative to spiral MR Fingerprinting1School of Engineering, Institute for Digital Communications, University of Edinburgh, Edinburgh, United Kingdom, 2Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom, 3Computer Science, Technische Universität München, Munich, Germany, 4GE Global Research, Munich, Germany
Synopsis
This study shows the practical implementation of an accelerated Cartesian Magnetic Resonance Fingerprinting (MRF) scheme using a multi-shot Echo Planar Imaging (EPI) readout. Its performance is compared with conventional spiral MRF and the fast convergence of accelerated iterative reconstructions for this method is shown.
Purpose
The main purpose of this study is to show that a highly accelerated Cartesian MRF scheme using a multi-shot EPI readout (i.e. multi-shot EPI-MRF) can produce good quality multi-parametric maps such as T1, T2 and proton density (PD) in a sufficiently short scan duration that is similar to conventional MRF1. This multi-shot approach allows considerable subsampling while traversing the entire k-space trajectory, can yield better SNR, reduced blurring, less distortion and can also be used to collect higher resolution data compared to existing single-shot EPI-MRF implementations2, 3. The generated parametric maps are compared to an accelerated spiral MRF implementation with the same acquisition parameters to evaluate the performance of this method4. Additionally, an iterative reconstruction algorithm is applied to improve the accuracy of parametric map estimations and the fast convergence of EPI-MRF is also demonstrated5.Methods
The scanning was performed on a 3T GE MR750w scanner with a 12 channel receive only head RF coil (GE Medical Systems, Milwaukee, WI). 16-shot EPI-MRF datasets and spiral datasets with 89 interleaves and golden angle rotations were acquired from a tube phantom (Diagnostic Sonar, Livingston, UK) consisting of tubes with different T1 and T2 values and a healthy volunteer using the inversion recovery (IR) prepared (Tinv = 18 ms) Quantitative Transient-state Imaging (QTI) sequence, using a linear ramp flip angle (FA) variation from 1° to 70° for 500 frames4. The Gx and Gy gradient of the multi-shot EPI trajectory were balanced (Fig. 1) to ensure that the residual magnetization remained constant for every shot and the crusher gradient (Gz) was applied to introduce gradient spoiling. The TR was set to 16 ms for all the datasets to enable comparison between multi-shot EPI-MRF and spiral MRF. The acquisition time for a single slice was 9 s. Both acquisitions had 22:5 x 22:5 cm Field of View (FOV), 128 x 128 matrix size, 1.3 mm in-plane resolution and 5 mm slice thickness. The reconstruction was performed by the CS based dictionary matching method with iterative reconstructions to generate quantitative T1, T2 and PD maps5, 6. The dictionaries were calculated using the Extended Phase Graph (EPG) model7.Results
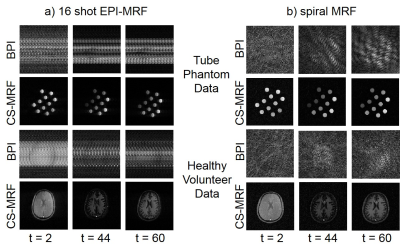
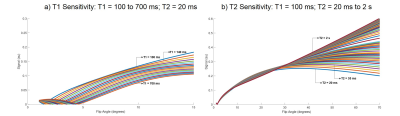
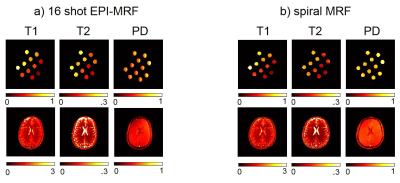
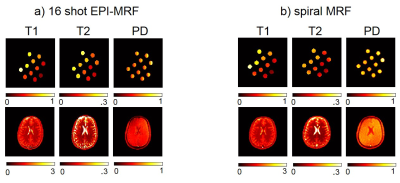
Fig. 2 shows the highly subsampled aliased images of the tube phantom and healthy volunteer at different frames/repetitions indexes t along with the CS-based reconstruction that removed the aliasing and provided a better visualization of the signal temporal dynamics. Fig. 3 shows the T1 and T2 sensitivity of the sequence for discriminating dictionary elements using a linear ramp FA variation from 1° to 70° for 500 frames. Fig. 4 shows the generated T1, T2 and PD maps for the tube phantom and healthy volunteer for both the 16 shot EPI-MRF and spiral MRF acquisitions. Fig. 5 shows the qualitatively improved parametric estimations of T1, T2 and PD maps for the tube phantom and healthy volunteer after the application of an iterative projection algorithm.Discussion and Conclusion
Fig. 3a shows that the T1 sensitivity is high throughout the acquisition, is enhanced by the initial inversion pulse and occurs mostly at lower flip angles whereas Fig. 3b shows that the T2 sensitivity occurs mostly at higher flip angles (> 25°). The T1 and T2 values of EPI-MRF and spiral MRF in Fig. 4 and Fig. 5 for grey matter (GM) and white matter (WM) are very similar to each other, in close agreement to those reported in literature8. However, there is an underestimation of cerebrospinal fluid (CSF) T2 values in both EPI-MRF and spiral MRF and slight aliasing artefacts also appear in the T2 and PD maps of EPI-MRF. This is because the encoding scheme used in the acquisition is comparatively less sensitive to T2 variations than T1 variations. The use of an optimized FA train instead of a linear ramp may yield more accurate T2 values and may potentially suppress the ghosting artefacts in the T2 and PD maps. Fig. 5 shows that the accuracy of the parametric maps was improved by the use of iterative reconstruction algorithms. The iterative reconstructions of multi-shot EPI-MRF converges very quickly (35 s) compared to spiral MRF (~ 4 minutes) and could therefore result in a very fast implementation on the scanner. This could be further improved by the use of an adaptive iterative algorithm9. The convergence of spiral acquisition is slow (which means more iterations) because spiral sampling is ill-posed10. Moreover, each iteration is more expensive because spiral sampling uses costlier non-uniform fast Fourier Transform (NUFFT) compared to FFT in EPI. In addition, higher resolution data can be acquired using a multi-shot EPI acquisition because it does not suffer from blurring artefacts that become more pronounced in spiral acquisitions at longer readout durations11.Acknowledgements
The research leading to these results has received funding from the European Union's H2020 Framework Programme (H2020-MSCA-ITN-2014) under grant agreement no 642685 MacSeNet and the Engineering and Physical Sciences Research Council (EPSRC) platform grant, number EP/J015180/1.References
[1] Y. Jiang, D. Ma, N. Seiberlich, V. Gulani, and M. A. Griswold, "MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout," Magnetic resonance in medicine, vol. 74, pp. 1621-1631, 2015.
[2] B. Rieger, F. Zimmer, J. Zapp, S. Weingärtner, and L. R. Schad, "Magnetic resonance fingerprinting using echo‐planar imaging: Joint quantification of T1 and T2∗ relaxation times," Magnetic resonance in medicine, vol. 78, pp. 1724–1733, 2017.
[3] O. Cohen, M. Sarracanie, M. S. Rosen, and J. L. Ackerman, "In Vivo Optimized Fast MR Fingerprinting in the Human Brain," in Proc Intl Soc Mag Reson Med, #430, 2016.
[4] P. A. Gomez, G. Buonincontri, M. Molina-Romero, J. I. Sperl, M. I. Menzel, and B. H. Menze, "Accelerated parameter mapping with compressed sensing: an alternative to MR Fingerprinting," in Proc Intl Soc Mag Reson Med, #1167, 2017.
[5] M. Davies, G. Puy, P. Vandergheynst, and Y. Wiaux, "A compressed sensing framework for magnetic resonance fingerprinting," SIAM Journal on Imaging Sciences, vol. 7, pp. 2623-2656, 2014.
[6] M. Davies, G. Puy, P. Vandergheynst, and Y. Wiaux, "Compressed quantitative MRI: Bloch response recovery through iterated projection," in Acoustics, Speech and Signal Processing (ICASSP), 2014 IEEE International Conference on, 2014, pp. 6899-6903.
[7] M. Weigel, "Extended phase graphs: dephasing, RF pulses, and echoes‐pure and simple," Journal of Magnetic Resonance Imaging, vol. 41, pp. 266-295, 2015.
[8] J. P. Wansapura, S. K. Holland, R. S. Dunn, and W. S. Ball, "NMR relaxation times in the human brain at 3.0 tesla," Journal of magnetic resonance imaging, vol. 9, pp. 531-538, 1999.
[9] M. Golbabaee, Z. Chen, Y. Wiaux and M. E. Davies,"Cover tree for fast MR fingerprint Recovery", Proceedings of the IEEE workshop on Machine Learning for Signal Processing (MLSP), 2017, arXiv:1706.07834.
[10] C. C. Cline, X. Chen, B. Mailhe, Q. Wang, J. Pfeuffer, M. Nittka, et al., "AIR-MRF: accelerated iterative reconstruction for magnetic resonance fingerprinting," Magnetic Resonance Imaging, vol. 41, pp. 29-40, 2017.
[11] K. T. Block and J. Frahm, "Spiral imaging: a critical appraisal," Journal of Magnetic Resonance Imaging, vol. 21, pp. 657-668, 2005.
Figures