4254
Dictionary free anatomical segmentation of Magnetic Resonance Fingerprinting brain data with Independent Component Analysis1Radiology, Case Western Reserve University, Cleveland, OH, United States, 2Radiology, University Hospitals, Cleveland, OH, United States
Synopsis
Magnetic Resonance Fingerprinting signal evolutions are sensitized to certain tissue properties during data acquisition. The matching step can be suboptimal due to dictionary limitations or tissue related constraints (e.g. partial volume, magnetization exchange). Here, we propose to apply Independent Component Analysis (ICA) to 4D MRF data after image reconstruction without explicit dictionary matching for tissue characterization, lifting the requirement for a relaxation model. ICA of whole brain MRF data segments the brain into multiple components with single tissue types such as gray matter, white matter and CSF for healthy subjects and also tumor in the case of glioblastoma patients.
Introduction
Magnetic Resonance Fingerprinting1 (MRF) is a quantitative tissue property and system parameter mapping technique consisting of three essential steps: data acquisition, dictionary construction and matching. First, in data acquisition, the MR signal is manipulated with variable flip angles and repetition times (TRs), and other dedicated modules (inversion pulses, T2 prep, etc.) to make MRF signal evolutions sensitive to a range of T1/T2 values and other desired tissue properties. Then, a dictionary with entries spanning all possible properties and parameters is constructed. The signal evolution from each voxel is then matched to a dictionary entry and thus to a certain tissue property value. Any source of variation available in MRF signal evolutions not addressed in the dictionary step is likely to affect the accuracy of matching process, especially if it is not orthogonal to any of other simulated tissue properties and system parameters. Also, when reduced to quantitative maps, the potentially valuable source of variation in the MRF time course might not be fully exploited. To explore the possibility of spatio-temporal analysis of 4D MRF data in a model independent manner, Independent Component Analysis (ICA) is applied to healthy subject and patients brain MRF data immediately after image reconstruction and without any dictionary matching.Methods
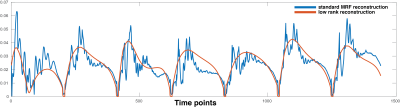
In the IRB approved study, a 3D MRF scan2,3 was performed in two healthy subjects and two patients diagnosed with glioblastoma (GBM). The acquisition parameters were FOV: 300x300x144, matrix size: 256x256x48, image resolution: 1.2x1.2x3 mm3, acquisition time: 4.6 minutes. kt-SVD low rank reconstruction4 was applied to MRF data to generate aliasing-free time series (Figure 1). 4D MRF data of two healthy subjects and two patients diagnosed with GBM were fed into ICA5 separately with automated dimensionality estimation. ICA is a blind source separation typically used for decomposing 4D (space x time) functional MRI data into multiple spatial independent components (IC) each with their associated time course. ICA applied to fMRI reveals groups of voxels that have similar signal time courses hypothesized to originate from simultaneous neuronal activity. In the proposed technique, underlying tissue properties cause the MRF signal to have a similar time course for a given tissue, and voxels which contain this tissue are expected to be grouped together.Results
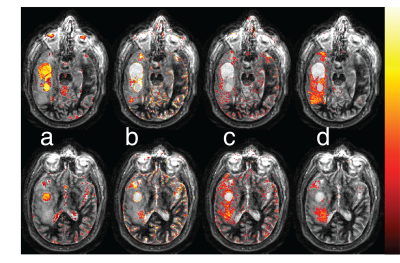
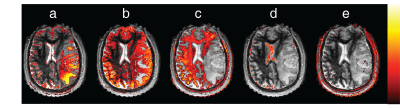
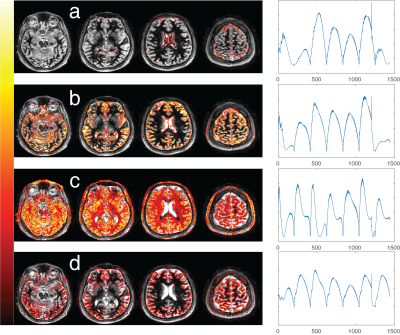
In our preliminary results, the components obtained from ICA of GBM patients and healthy subjects mostly correspond to anatomical structures. Figure 2 shows spatial maps of 4 ICs associated with both the solid tumor region and the peri-tumoral white matter region from the first GBM patient ICA. For comparison, Figure 3 has components containing normal tissue as well as the solid tumor for the second GBM patient. In both figures, tumor associated ICs partially also include cerebrospinal fluid (CSF) and grey matter tissue in their spatial maps. White matter components, on the other hand, do not overlap with tumor regions at all. Time courses of ICs found in all datasets is illustrated for one healthy subject in Figure 4. Figure 5 illustrates gray matter associated component from a healthy subject’s ICA for the whole brain.Discussion
Here we have demonstrated that potentially useful maps of tissue structure can be generated from MRF data without using an explicit dictionary matching step. Several steps must be undertaken in order to observe the signal differences. Since ICA looks for similar time courses across space, it can also be employed for detection of noise sources with structured time signals. In fact, when ICA is run on standard MRF time course reconstructions with the intent of separating noise and tissue simultaneously, almost all ICs belong to the aliasing artifacts with more than 95% of the variation in total. Only after low rank reconstruction is one able to reach the small variations caused underlying tissue properties. Experimentally it is found that ICA with automated estimation of dimensionality provide an acceptable measure for the degrees of freedom with ~10 structurally meaningful ICs.
The time courses of ICs shown in Figure 4 also give insights for the optimization of MRF data acquisition for ICA. Gray matter and CSF mostly differ in the beginning of the acquisition and after the inversion pulse at time point 1200, suggesting T1 as the dominant property separating these tissues. White matter time course is different throughout the whole time series, suggesting both T1 and T2 sensitivity.
Conclusions
ICA applied to brain MRF data results in consistent anatomical segmentation of brain tissue for healthy subjects and GBM patients. The proposed exploratory analysis of MRF time course data is dictionary free and does not rely on a relaxation model.Acknowledgements
The authors would like to acknowledge funding from Siemens Healthcare, NIH grants 1R01EB016728, 1R01DK098503, 1R01BB017219.References
1. Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature 2013;495: 187–192.
2. Ma D, Pierre E, McGivney D, et al. Applications of Low Rank Modeling to Fast 3D Magnetic Resonance Fingerprinting. ISMRM Proceedings 2017; p129.
3. Ma D, Jiang Y, Chen Y, et al. Fast 3D magnetic resonance fingerprinting for a whole-brain coverage. Magn Reson Med 2017; doi:10.1002/mrm.26886.
4. Pierre E, Griswold M, Connelly A. Fast Analytical Solution for Extreme Unaliasing of MR Fingerprinting Image Series. ISMRM Proceedings 2017; p1353.
5. Melodic, FSL, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC
Figures