4249
Time efficient whole-brain coverage in Magnetic Resonance Fingerprinting using echo-planar imaging, slice-interleaving and simultaneous multi-slice imaging1Computer Assisted Clinical Medicine, Heidelberg University, Mannheim, Germany, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 3Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States
Synopsis
Magnetic resonance fingerprinting (MRF) is a promising method for simultaneous multi-parameter quantification. However, spatial coverage is limited in the original method due to the rapid image readout and the balanced sequence design. The aim of this study is to enable time-efficient volumetric coverage in Cartesian MRF-EPI for whole-brain quantification in clinically acceptable scan times. A slice-interleaved acquisition is shown to offer quantification precision comparable to single-slice measurements in a fraction of the measurement time. By combining this with simultaneous multi-slice imaging, whole-brain coverage with 40 slices was achieved in one minute, resulting in high quality T1 and T2* maps.
Introduction
Magnetic resonance fingerprint (MRF) has been introduced with great promise for simultaneous quantification of a variety of parameters such as such at T1, T2 and T2*1. Although it has been mainly limited to single slice implementations, first 3D implementations based on stack of spiral readout have recently been suggested. Initial studies propose scan time of multiple minutes for whole brain imaging2,3, despite elaborate regularization to allow image reconstruction from highly undersampled 3D k-spaces. The purpose of this work is to increase scan-time efficiency of MRF with improved spatial coverage by integrating a slice-interleaved acquisition scheme in MRF-EPI4. Scan times are further reduced by employing simultaneous multi-slice imaging, ultimately enabling whole brain coverage in less than one minute.Methods
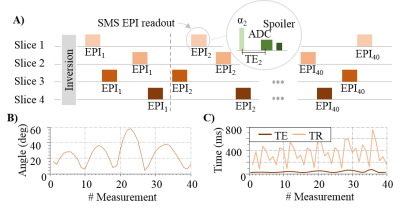
The previously proposed MRF-EPI4 sequence was adapted to integrate a slice-interleaved acquisition scheme (Fig. 1). Following an initial global inversion pulse to increase T1 sensitivity, numerous EPI readouts of multiple slices are acquired in a interleaved manner. Flip-angle and TE of the readouts is varied and the slice-order is pseudo-randomized to induce additional TR variation (Figure 1c). The overall number of image acquisitions was kept constant. Thus, an increased number of slices leads to a decreased number of baseline images per slice, although at increased SNR due prolonged effective slice TR. The optimal number of interleaved slices was determined studying the noise amplification in an MRF experiment4. For whole-brain coverage, multiple slice-interleaved measurements were performed successively, separated by 10s. Dictionary entries were generated by Bloch-equation simulations on a per-slice basis, including B1+ correction. Slice-interleaved MRF-EPI was tested in phantoms and 6 healthy volunteers (4 men, 31±6 years) on a 3T scanner (Siemens) with a 32 channel head coil: 4 slices per slice group, TE/TR=17-78ms/288-755ms, flip angle=4-58°, FOV=220×220×140mm3, slice-gap=0.9mm, voxel size=1.0×1.0×3.0mm3, band-width=1136Hz/pixel, partial-Fourier=5/8, in-plane acceleration 3 with GRAPPA reconstruction, acquisition time per slice group=17s, total number of baseline images=160 (4 slices with each 40 images). The quantification accuracy was validated in phantom scans against IR-TSE for T1 and GRE for T2*. Highly accelerated whole-brain in-vivo MRF quantification was performed in one healthy volunteer (male, 29 years) by additionally employing SMS acceleration: SMS-acceleration=3-5, 64 channel head coil, TE/TR=27-27ms/125-722ms, in-plane acceleration=2 (for SMS=5).Results
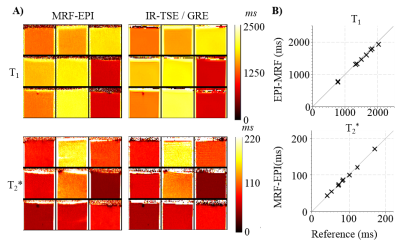
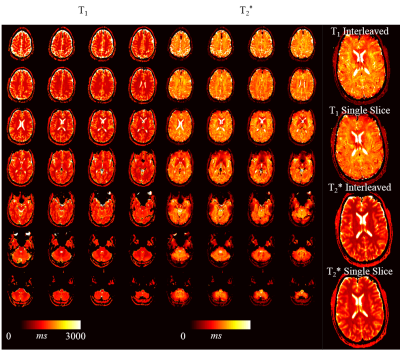
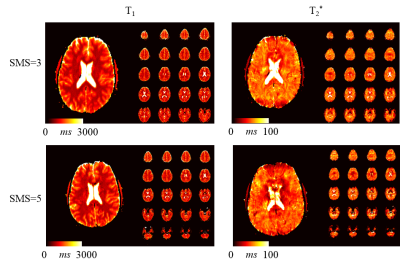
Simulations show that the average noise amplification is almost constant between 40 and 160 baseline images. Thus, 4 slices were chosen for interleaved acquisitions resulting in the highest acceleration factor with marginal loss of precision. Phantom results depict homogeneous T1 and T2* estimates with the proposed method. Good quantitative agreement with the reference measurements are observed, with slight, residual underestimation of long T1 times (T1: -2.4±1.1%, T2*:-0.5±1.5%). Full brain MRF data was successfully acquired in 3:36min, providing visually high parameter map quality, with good white/gray matter delineation in the T1 maps, and susceptibility weighted contrast in the T2* maps (Figure 4). The maps are visually comparable to single-slice measurements. Exemplary fingerprints of a highly accelerated sliced-interleaved scan with SMS acceleration of 5 demonstrates that signal paths are distinctly different from various brain regions, despite the reduced number of baseline images. SMS accelerated slice-interleaved MRF maps were successfully acquired in 1:12min (40slices, SMS=5) and 1:10s (24slices, SMS=3). For both acquisitions the corresponding T1 maps are visually comparable to the slice-interleaved scheme. At SMS=5 slight, residual artifacts are apparent in the T2* maps (Figure 5).Discussion
Slice-interleaved MRF-EPI method showed increased scan time efficiency by a factor of 4 compared to single-slice MRF in this study. Our results indicate that reducing the number of baseline images has marginal effect on the quantification precision, as longer effective slice TR lead to higher baseline SNR. Integrating SMS into the slice-interleaved method further decreases scan time, enabling whole-brain in-vivo quantification within 1:12 minutes. Presently, relaxation periods are required between different slice-groups due to the application of a non-selective inversion-pulse. This can potentially be circumvented using multi-banded slice-selective inversion-pulses. At SMS factor of 5, in-plane acceleration had to be adjusted to allow for effective FOV shifting using CAIPIRINHA. In-vivo T2* maps acquired at this acceleration show residual artifacts in the T2* maps, likely due to the increased readout duration. Optimized phase shift patterns are to be explored in future studies, to enable SMS 5 with higher in-plane acceleration.Conclusion
In this study we have shown that slice-interleaved MRF-EPI with SMS allows for time efficient simultaneous T1 and T2* mapping, with whole-brain coverage in one minute. Slice-interleaved implementation shows comparable quantification precision to single-slice measurements in a fraction of the measurement time.Acknowledgements
No acknowledgement found.References
1. Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature 2013;495:187–192. doi: 10.1038/nature11971.
2. Liao C, Bilgic B, Manhard MK, Zhao B, Cao X, Zhong J, Wald LL, Setsompop K. 3D MR fingerprinting with accelerated stack-of-spirals and hybrid sliding-window and GRAPPA reconstruction. NeuroImage 2017;162:13–22. doi: 10.1016/j.neuroimage.2017.08.030.
3. Ma D, Jiang Y, Chen Y, McGivney D, Mehta B, Gulani V, Griswold M. Fast 3D magnetic resonance fingerprinting for a whole-brain coverage. Magn. Reson. Med.:n/a-n/a. doi: 10.1002/mrm.26886.
4. Rieger B, Zimmer F, Zapp J, Weingärtner S, Schad LR. Magnetic resonance fingerprinting using echo-planar imaging: Joint quantification of T1 and T2∗ relaxation times. Magn. Reson. Med. 2016:n/a-n/a. doi: 10.1002/mrm.26561.
Figures