4238
Efficient imaging of Rabi modulated trajectories using balanced Steady-State Driven Trajectory (bSSDT) imaging1Dept. Biomedical Engineering, University of Melbourne, Melbourne, Australia, 2Dept. Electrical and Computer Systems Engineering, Monash University, Melbourne, Australia, 3Dept. of Medical Physics and Biomedical Engineering, Shiraz University of Medical Sciences, Shiraz, Iran (Islamic Republic of), 4Dept. Electrical and Electronic Engineering, University of Melbourne, Melbourne, Australia
Synopsis
We propose an efficient technique to image localised steady-state trajectories, termed balanced Steady-State Driven Trajectory (bSSDT) imaging and implement the protocol to investigate the properties of Rabi modulated steady-state trajectories. In bSSDT imaging, a sequence of points on the voxel-wise steady-state trajectory is acquired. The resultant 4-D data offers a potentially rich source of information about the underlying tissue properties. The proposed imaging technique is a pseudo-continuous excitation version of balanced steady-state free precession (bSSFP) imaging, with relaxation of the magnetisation to the equilibrium steady-state in bSSFP replaced by control of the magnetisation to a steady-state trajectory in bSSDT.
Introduction
Rabi modulated continuous wave excitation drives the bulk magnetisation into a predictable harmonic steady-state trajectory, the shape and form of which are governed by both the sequence and tissue parameters1,2. The information-rich Rabi steady-state trajectories have previously been used to encode off-resonance information and applied to proof-of-concept spectroscopy3 and imaging protocols4. Here, we propose an efficient technique to acquire image data of the localised steady-state trajectories, termed balanced Steady-State Driven Trajectory (bSSDT) imaging. This is in direct comparison with balanced Steady-State Free Precession (bSSFP) imaging5, in which the steady-state is centred around equilibrium rather than driven into a Rabi steady-state trajectory. The bSSDT technique acquires a sequence of points on a steady-state trajectory (4-D data) rather than a single point (3-D data) as acquired by bSSFP sequences, offering potentially greater insight into the underlying tissue properties.Methods
Experiments were conducted on a 4.7T Bruker Biospec scanner with an AVANCE III console. An imaging phantom with three test tubes of Gadolinium doped distilled water was aligned along the longitudinal axis.
Balanced steady-state driven trajectory (bSSDT) imaging:
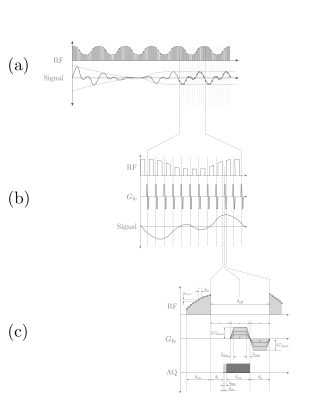
A gapped excitation measurement protocol6 (Fig. 1a), similar to that used in SWIFT7, was used to achieve near-simultaneous transmit and receive with an 80% duty cycle. A balanced frequency encoding gradient was applied during the free induction decay period to acquire a $$$k$$$-space spoke. The bSSDT sequence is similar to bSSFP5 in the use of a balanced gradient to maintain a stable magnetisation, however bSSDT measures a steady-state trajectory rather than a single steady-state point.
RF Excitation: A Rabi modulated excitation envelope, $$\gamma B_\textrm{1}^\textrm{e}\left(t\right) = \omega_\textrm{1} \left( 1 + \alpha \cos{\omega_\textrm{1} t}\right)$$ was used, where $$$\alpha=2$$$ is the modulation depth and $$$\omega_\textrm{1}=45$$$ Hz is both the average excitation strength and the envelope modulation frequency.
Acquisition: The Rabi steady-state trajectory (2 cycles, 10 points per cycle) was measured over a 3D volume (FOV=64mm, 1mm isotropic, nProj=12753) in approximately 10 mins. Each radial-out spoke was acquired in 149$$$\mu$$$s with a max gradient of 127mT/m (25% system max) in order to satisfy the geometry, steady-state sampling and RF envelope parameters. The digitiser was turned on before the gradients to monitor the stability of the steady-state trajectory.
Reconstruction: A 3D volume was reconstructed from the radial $$$k$$$-space data at each steady-state timepoint. Density compensation8 and re-gridding9,10 of the $$$k$$$-space data was achieved using routines from the MRI Unbound project11. The re-gridded $$$k$$$-space data was then Fourier transformed into the image domain.
Reference data:
UTE3D imaging: A reference image was acquired with the UTE3D sequence (FOV=64mm, 1mm isotropic, TE=8.13$$$\mu$$$s, TR=4ms, nProj=12753) and reconstructed with a measured and a theoretical $$$k$$$-space trajectory.
Relaxometry: T$$$_\textrm{1}$$$ maps were acquired using RARE-VTR (1 slice, 2mm thickness, FOV=64mm, 128x128 matrix, TE=40ms, TR=12500, 2212, 500ms, RARE factor 4). T$$$_\textrm{2}$$$ maps were acquired using MSME (250 echoes, 20ms echo spacing, 1 slice, 2mm thickness, FOV=64mm, 64x64 matrix, TR=12.5s).
Field mapping: B$$$_\textrm{0}$$$ field maps were measured using a multiple gradient echo method12 (TE=1.89, 6.17ms, FA=30$$$^\circ$$$, TR=20ms, FOV=64mm, 64x64x64 matrix). B$$$_\textrm{1}$$$ field maps were measured using a double angle method13 (64 slices, 1mm thickness, FOV=64mm, 64x64 matrix, TE=20ms, TR=12.5s) with excitation and refocusing angles ($$$\alpha_\textrm{1}$$$/$$$\beta_\textrm{1}$$$=45$$$^\circ$$$/90$$$^\circ$$$ and $$$\alpha_\textrm{2}$$$/$$$\beta_\textrm{2}$$$=90$$$^\circ$$$/180$$$^\circ$$$).
Voxel trajectory prediction:
To evaluate the accuracy of bSSDT, theoretical Rabi steady-state trajectories for each voxel were calculated via harmonic balancing1 using the measured relaxation and field maps.
Results & Discussion
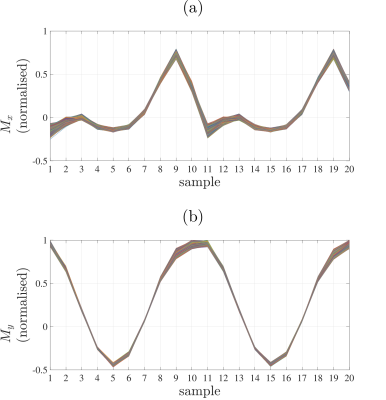
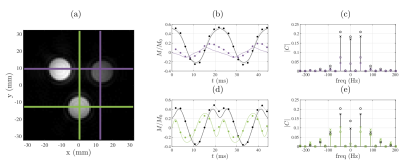
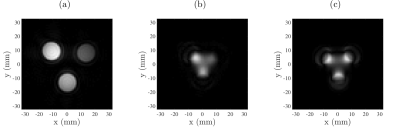
The stability of the navigator data acquired before each $$$k$$$-space spoke (Fig. 2) demonstrates the ability to maintain a steady-state trajectory using balanced gradients. The measured voxel steady-state trajectories (Fig. 3) show good agreement with the predicted steady-state and highlight the sensitivity of the Rabi steady-state to relaxation effects and magnetic field variation. At this point in development, the bSSDT reconstruction (Fig. 4) suffers from a distortion and edge artifact similar to that observed in the UTE3D reconstruction without a measured $$$k$$$-space trajectory correction. The bSSDT reconstruction artifact will be resolved in the near future by incorporating $$$k$$$-space measurement14.
As Rabi steady-states are sensitive to off-resonance, excitation field strength and relaxation effects, we anticipate that modulation of the Rabi excitation parameters in bSSDT will countenance the joint estimation of magnetic fields and spin-system properties from a single acquisition, akin to Magnetic Resonance Fingerprinting15.
Conclusion
We have experimentally demonstrated an efficient method to image Rabi modulated steady-state trajectories, termed balanced Steady-State Driven Trajectory imaging. Our method exploits the non-zero steady-state magnetisation that emerges from Rabi modulated continuous wave excitation of a spin system. It is of interest to investigate the image contrast achievable using the voxelwise measured steady-state trajectories that encode the properties of the tissue.Acknowledgements
We acknowledge the facilities, and the scientific and technical assistance of the Australian National Imaging Facility at the Melbourne Brain Centre Imaging Unit. The Australian National Imaging Facility is funded by the Australian Government NCRIS program.References
- Tahayori B, Johnston L, Layton K, Farrell P, Mareels I. Solving the Bloch equation with periodic excitation using harmonic balancing: Application to Rabi modulated excitation. IEEE Transactions on Medical Imaging. 2015;34(10):2118-2130.
- Layton KJ, Tahayori B, Mareels IM, Farrell PM, Johnston LA. Rabi resonance in spin systems: Theory and experiment. Journal of Magnetic Resonance. 2014;242:136-142.
- Korte JC, Layton KJ, Tahayori B, Farrell PM, Moore SM, Johnston LA. NMR spectroscopy using Rabi modulated continuous wave excitation. Biomedical Signal Processing and Control. 2017;33:422-428.
- Korte JC, Tahayori B, Farrell PM,
Moore SM, Johnston LA. Rabi modulated continuous wave imaging. In Proceedings
of the 24th Annual Meeting of ISMRM, Singapore. 2016.
- Oppelt A, Graumann R, Barfuss H, Fischer H, Hartl W, Schajor W. FISP-a new fast MRI sequence. Electromedica. 1986;54(1): 15-18.
- Korte JC, Tahayori B, Farrell PM, Moore SM, Johnston LA. Gapped measurement of spin system response to periodic continuous wave excitation. ANZMAG, Bay of Islands, New Zealand. 2015.
- Idiyatullin D, Corum C, Park JY, Garwood M. Fast and quiet MRI using a swept radiofrequency. Journal of Magnetic Resonance. 2006;181(2):342-349
- Zwart NR, Johnson KO, Pipe JG. Efficient sample density estimation by combining gridding and an optimized kernel. Magnetic Resonance in Medicine. 2012;67(3):701-710.
- Jackson JI, Meyer CH, Nishimura DG, Macovski A. Selection of a convolution function for Fourier inversion using gridding. IEEE Transactions on Medical Imaging. 1991; 10(3):473-478.
- Beatty PJ, Nishimura DG, Pauly JM. Rapid gridding reconstruction with a minimal oversampling ratio. IEEE Transactions on Medical Imaging. 2005;24(6):799-808.
- Zwart NR, Turley DC, Johnson KO, Pipe JG, Sampling density calculation and re-gridding routines, www.ismrm.org/mri_unbound/sequence.htm
- Kanayamay S, Kuhara S, and Satoh K. In vivo rapid magnetic field measurement and shimming using single scan differential phase mapping. Magnetic Resonance in Medicine. 1996;36(4):637.
- Stollberger R, Wach P, McKinnon G, Justich E, and Ebner F. RF-field mapping in vivo. In Proceedings of the 7th Annual Meeting of SMRM. 1988;page 106.
- Addy NO, Wu HH, Nishimura DG. Simple method for MR gradient system characterization and k‐space trajectory estimation. Magnetic Resonance in Medicine. 2012;68(1):120-129.
- Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature. 2013;495(7440):187-192.
Figures