4236
Reducing Blurring while Controlling Contrast in Abdominal Imaging with Variable Flip Angle Single Shot Fast Spin Echo1Radiology, Beth Israel Deaconess Medical Center & Harvard Medical School, Boston, MA, United States, 2GE Healthcare, Global MR Applications and Workflow, New York, NY, United States
Synopsis
Single-shot fast spin echo is used clinically to provide fast, motion-robust abdominal images. However, signal decay along the echo train results in blurred images. Our previous work on optimal variable flip angle design demonstrated improved SNR on brain imaging. Here, we extend this framework to show simultaneous T2 blurring and contrast control in abdominal imaging with single-shot fast spin echo.
Purpose
Single-shot fast spin echo is routinely used in clinical abdominal imaging because of its motion robustness and compatibility with breath-hold imaging. However, the long echo train results in T2 blurring and scan time is limited by high specific absorption rates (SAR). Variable flip angle (VFA) designs have been proposed to increase speed (1) and to reduce T2 blurring (2). However, existing VFA design has three limitations. First, it was primarily designed to reduce SAR without simultaneous control of T2 blurring. Second, the contrast is determined by phase encoding order, hence not taking advantage of the flexible contrast that could be designed with VFA. Third, conventional VFA is designed in a heuristic way, based on the user experience and may result in suboptimal result.
We previously proposed a framework to design VFA as an optimization problem and demonstrated its ability by 40% SNR improvement in T2 blurring correction (3). Here, we extend this framework to provide simultaneous control of T2 blurring and contrast in abdominal imaging.
Methods
In this work, VFA is designed to maximize SNR while controlling T2 blurring with a k-space filter correction and maintaining the contrast. Because spleen and liver metastases have similar T2 and proton densities (4), the proposed method is designed to improve liver and spleen image quality in healthy subjects to enable better detection of liver metastases in the future.
The signals of the liver and spleen are $$$S_{liver}$$$ and $$$S_{spleen}$$$ during the echo train. The tissue contrast can be defined as:
$$ \frac{S_{liver}(k) - S_{spleen}(k)}{S_{liver}(k) + S_{spleen}(k)}>c $$
Where the kth echo is acquired at the k-space center, c is the desired contrast (0.3 in this study).
The target of the optimal VFA design framework (3) is to maximize SNR when the signal of the liver is corrected to a k-space filter W, for example, a Hanning window in this study.
$$ SNR \propto \frac{1}{\sigma}\frac{\sum{W_i}}{\sqrt{\sum{(\frac{W_i}{S_{liver}})^2}}} $$
$$ \hat{S}=argmin \sum{(\frac{W_i}{S_{liver}})^2} $$
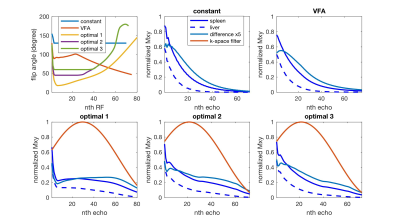
In this work, we proposed three flip angle schemes, referred to as optimal 1, 2 and 3, which constrains the minimum flip angle to 0, 45 and 60 degrees, respectively. T1, T2 and relative proton density were set to 1328ms, 61ms, and 1 for the spleen, and to 809ms, 34ms and 0.7 for the liver (5).
Two conventional flip angle designs were also acquired as references: a constant amplitude flip angle design with flip angle 130 degrees, named ‘constant’, and a previously published variable flip angle design (6), named ‘VFA’.
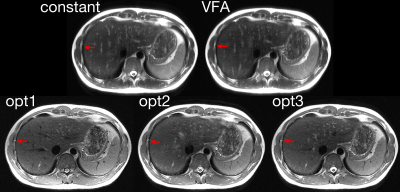
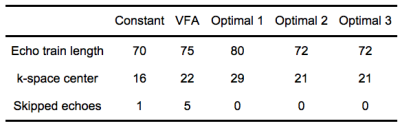
Experiments were performed on a GE 3T scanner A single-slice of the liver was acquired within a breath-hold. Image size was 382x224 and echo spacing was 4.8ms in VFA. Parallel imaging was used to reduce the echo train length by a factor of 2.5. Echo-train characteristics for each design are listed in Table 1.
Data were corrected to k-space filter W and reconstructed in MATLAB 2017a. Liver and spleen regions were selected manually on the ‘constant’. Noise level was measured as the standard deviation of the background and SNR was calculated as the ratio between liver signal and background noise. Image blurring was quantified by a non-reference method (7), where low values represent sharp images. The contrast between spleen and liver was calculated.
Results and Discussion
The contrast between spleen and liver was consistent between the design (>0.3) and the experiment (0.31, 0.30 and 0.33 for the optimal designs 1, 2 and 3).
Compared to the ‘constant’ (blurring=0.2, SNR=25.7) and ‘VFA’ (blurring=0.21, SNR=26.9), the proposed methods reduced the blurring (blurring of optimal 1/2/3=0.16/0.17/0.16) with a slight SNR penalty (SNR of optimal 1/2/3=23.9/25.0/24.2). Reduced blurring on the edges of the liver is highlighted in Figure 2. This improvement is due to the more uniformly distributed echo signals across k-space in the proposed methods, Figure 1, which reduced the high-frequency noise amplification in the k-space filter correction.
The optimal 1 design also suppressed the signal of blood in large vessels and CSF in the spinal canal due to the use of low flip angles. However, it increased motion-sensitivity and B1 inhomogeneity, which could result in motion and dielectric artifacts. The flow suppression was reduced when higher flip angles were used in the optimal 2 and 3 designs.
Conclusion
This work demonstrated simultaneous contrast and resolution control with an optimal framework of variable flip angle design for fast spin echo. The proposed methods provided superior spatial resolution with slightly reduced SNR, compared to conventional constant amplitude and existing variable flip angle designs. This framework can be extended to general echo train based acquisitions and offers flexible control of contrast, blurring and power deposition.Acknowledgements
No acknowledgement found.References
1. Loening AM, Saranathan M, Ruangwattanapaisarn N, Litwiller D V., Shimakawa A, Vasanawala SS. Increased speed and image quality in single-shot fast spin echo imaging via variable refocusing flip angles. J. Magn. Reson. Imaging 42:1747–1758. doi: 10.1002/jmri.24941.
2. Leoning AM, Litwiller D V., Saranathan M, Vasanawala SS. Increased Speed and Image Quality for Pelvic Single-Shot Fast Spin-Echo Imaging with Variable Refocusing Flip Angles and Full-Fourier Acquisition. Radiology 2017;282:561–568. doi: 10.1148/radiol.2016151574.
3. Zhao L, Alsop DC. Combined flip angle and echo scaling modulation for optimal fast spin echo. Proc. Intl. Soc. Mag. Reson. Med. 2017:1325.
4. Van Lom KJ, Brown JJ, Perman WH, Sandstrom JC, Lee JK. Liver imaging at 1.5 tesla: pulse sequence optimization based on improved measurement of tissue relaxation times. Magn Reson Imaging 1991;9:165–171.
5. de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: Preliminary results. Radiology 2004;230:652–659. doi: 10.1148/radiol.2303021331.
6. Busse RF, Brau ACS, Vu A, Michelich CR, Bayram E, Kijowski R, Reeder SB, Rowley HA. Effects of refocusing flip angle modulation and view ordering in 3D fast spin echo. Magn. Reson. Med. 2008;60:640–649. doi: 10.1002/mrm.21680.
7. Crete-Roffe F, Dolmiere T, Ladret P, Nicolas M. The Blur Effect : Perception and Estimation with a New No-Reference Perceptual Blur Metric. SPIE Electron. Imaging Symp. Conf Hum. Vis. Electron. Imaging 2007:EI 6492-6416.