4229
Rapid multi-inversion SMS-EPI integrated with gradient-echo, spin-echo and diffusion-weighted EPI acquisitions1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Montreal Neurological Institute and Hospital, McGill University, Montréal, QC, Canada, 4Harvard-MIT Health Sciences and Technology, Institute of Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Recently inversion-recovery techniques have been combined with EPI in an efficient scheme in which a non-selective inversion is merged with a slice reordering scheme, providing rapid quantitative T1 maps. Here we merge this approach with several recent EPI technologies, including SMS-EPI, and several image contrasts including T2-, T2*, and diffusion weighted acquisitions. Not only does this allow the efficient combination of multiple contrasts but it opens the door to a rapid, all-EPI examination.
Introduction
T1 mapping provides insights into healthy and diseased states of the brain. However, the gold standard T1 mapping method is too long for many in vivo applications. Faster but less sensitive methods, such as the Look-Locker method, have been proposed as faster T1 mapping alternatives.
Higher temporal sampling of the T1 curve using 2D EPI acquisitions after a non-selective inversion pulse have been demonstrated previously [1-4]. In this work, we demonstrate three methods of 2D echo-planar imaging (EPI) acquisition following a non-selective inversion pulse built on top of modern product sequences with advanced functionality such as blipped-CAIPI SMS[5] and FLEET-ACS[6]: gradient-echo (GE) EPI, spin-echo (SE) EPI, and diffusion EPI. T1 maps acquired with the different techniques will have distortions that match fMRI, SE, and diffusion data respectively.
Gradient-echo T1 maps can be acquired quickly. However, they suffer from image distortion and signal loss due to B0 inhomogeneity; especially at high field.
Spin-echo T1 maps reduce that signal loss and image-distortion.
An interesting application of the IR-prepared diffusion is the ability to disentangle the contribution to T1 (related to myelin content) of multiple fiber populations within a voxel. This represents a significant improvement on other myelin mapping techniques, which can only measure total myelin content within a voxel.. This represents a significant limitation since crossing fibre configurations occur in 60-90% of voxels in the human brain at typical resolutions. By combining IR with diffusion, we can measure the T1 of individual fibre tracts. [7].
Methods
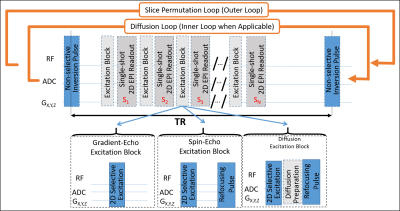
Common 2D EPI sequence framework: : Three similar product 2D EPI-readout based sequences (GE, SE, and diffusion) were modified to include a non-selective B0- and B1-insensitive FOCI inversion pulse [12] followed by a series of 2D EPI slices, each with unique inversion times. The inversion pulse followed by the series of 2D EPI slices were repeated multiple times (see outer loop in Figure 1) with the slice order permuted by shifting the slices by a constant shift, which can be greater than 1 for improved efficiency.
Advanced features in the sequence include blipped-CAIPI SMS [5], FLEET-ACS [6], LeakBlock [8], dynamic phase correction [9], and many other standard in-plane acceleration techniques available in the product sequence; also, a template matching framework amenable to fast dictionary matching.
In vivo experiments: A subject was scanned with all 3 sequence variants following informed consent and a Massachusetts General Hospital IRB approved protocol. Whole brain coverage was achieved with 1.5 mm (GE, SE) and 1.7 mm (Diffusion) isotropic in-plane resolution with slice thicknesses of 1.5 mm (GE,SE) and 2 mm (Diffusion). Slices were acquired with SMS 2 and a skip factor of 4. Multiple-Inversion diffusion was acquired in 24min. Spin echo was acquired in 5min and gradient echo images were acquired in 3min.
A subject was also scanned at McGill University with the multi-inversion diffusion EPI sequence following an institutionally approved protocol and informed consent.
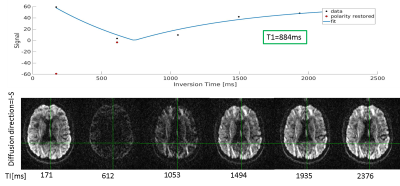
T1 Fitting: Signals were matched to precomputed signal models (or exponential recovery curve when TR was sufficiently long [10]) based on the expected range of T1 values while minimizing the error. Figure 2 shows an example of a single voxel fit.
Discussion/Conclusion
Here we have demonstrated the use of multi-inversion EPI for multiple image contrasts, which can be used as components of a rapid, all-EPI examination with matching geometric distortion. While our slice-permutation scheme provides rapid T1 quantification, motion correction of the EPI data can be challenging in multi-contrast acquisitions due to temporally-varying contrast. Future work will translate these efforts to ultra-high fields, where considerations such as SAR limits will have to be addressed. Fat saturation was used in the data presented here, but has been shown affects T1 quantitation [1], so water excitation may improve quantitative accuracy. Combining quantitative T1 mapping with diffusion-weighted imaging could be of potential interest in structural connectome studies as myelin modulates information flow through the networks of fiber tracts. Future directions include incorporating optimized ordering for improved temporal efficiency [11].Results
Fig. 3 demonstrates the MI-GE-EPI T1 map.
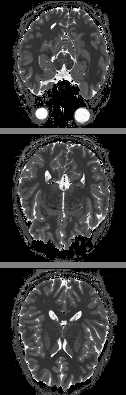
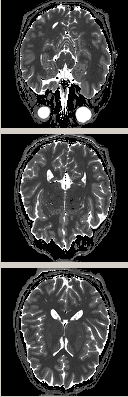
Fig. 4 presents an example of the MI-SE-EPI, which has reduced T2* contamination and through-plane signal dropout compared to the previously described MI-GE-EPI.
Fig. 5 demonstrates the MI-EPI diffusion weighted
acquisition, which merges diffusion weighting with inversion recovery in a
time-efficient manner.
Acknowledgements
This work was supported in part by the NIH NIBIB (grants P41-EB015896, and R01-EB019437), by the BRAIN Initiative (NIH NIMH grant R01-MH111419), and by the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging; and was made possible by the resources provided by NIH Shared Instrumentation Grants S10-RR023401 and S10-RR023043.References
1. Renvall V, Witzel T, Wald LL, Polimeni JR. Automatic cortical surface reconstruction of high-resolution T1 echo planar imaging data, Neuroimage. 2016; 134: 338–354.
2. Dougherty R, Mezer A, Zhu K, Kerr A, Middione M. Fast quantitative T1 mapping with simultaneous multi-slice EPI. Annu. Meet. Organ. Hum. Brain Mapp. 2014; 20:2002.
3. Grinstead JW, Wang D, Bhat H, Deshpande V, Cauley SF, Setsompop K, Benner T, Anderson VC, Rooney WD. Slice-accelerated inversion recovery T1 mapping. Proc. Int. Soc. Magn. Reson. Med. 2014; p3215.
4. Wu H, Tian Q, Poetter C, Zhu K, Middione MJ, Kerr AB, McNab JA, and Dougherty RF. Whole Brain Inversion Recovery Diffusion Weighted Imaging Using Slice-Shuffled Acquisition. Int. Soc. Magn. Reson. Med. 2017; p387.
5. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL., Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012 May;67(5):1210-24. PMID: 21858868.
6. Polimeni JR, Bhat H, Witzel T, Benner T, Feiweier T, Inati SJ, Renvall V, Heberlein K, Wald LL. Reducing sensitivity losses due to respiration and motion in accelerated echo planar imaging by reordering the autocalibration data acquisition. Magn Reson Med. 2016; 75(2):665-679. PMID: 25809559.
7. De Santis S, Barazany D, Jones DK and Assaf Y. Resolving Relaxometry and Diffusion Properties Within the Same Voxel in the Presence of Crossing Fibres by Combining Inversion Recovery and Diffusion-Weighted Acquisitions. Magn Reson Med. 2016; 75:372-380.
8. Cauley SF, Polimeni JR, Bhat H, Wald LL, Setsompop K. Interslice leakage artifact reduction technique for simultaneous multislice acquisitions. Magn Reson Med. 2014; 72(1):93-102. PMID: 23963964.
9. Setsompop K, Cohen-Adad J, Gagoski BA, Raij T, Yendiki A, Keil B, Wedeen VJ, Wald LL. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. Neuroimage. 2012; 63(1):569-580. PMID: 22732564.
10. Barral JK, Gudmundson E, Stikov N, Etezadi-Amoli M, Stoica P, Nishimura DG. Magn Reson Med. 2010 Oct;64(4):1057-67. doi: 10.1002/mrm.22497.
11. Cohen O and Polimeni JR. Optimized inversion-time schedules for quantitative T1 measurements based on high-resolution multi-inversion EPI. Magn Reson Med. August 27, 2017; PMID: 28845547.
12. Hurley AC, Al-Radaideh A, Bai L, Aickelin U, Coxon R, Glover P, Gowland PA. Tailored RF pulse for magnetization inversion at ultrahigh field. Magn Reson Med. 2010; 63(1):51-58. PMID: 19859955.
Figures