4205
Concomitant Field Compensation Improves Quality of Clinical Fast Spin Echo Images Acquired on an Asymmetric MRI Gradient System1Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
The fast-spin-echo (FSE) acquisitions are the workhorse for routine MRI, but can be affected by concomitant field (CF)-induced phase errors. Recently, a compact 3T (C3T) MRI platform equipped with an asymmetric, high-performance gradient was developed. The asymmetric design of this gradient induces zeroth/first-order CFs (in addition to the second-order CF on conventional whole-body gradients), which can degrade image quality of FSE acquisitions. We have previously developed real-time gradient pre-emphasis and frequency shifting techniques to compensate for these additional CFs. Here, we demonstrate that these compensations significantly improve image quality of FSE using a clinical T2w-FSE protocol and a preliminary dataset.
Purpose
Fast-spin-echo (FSE) or turbo-spin-echo (TSE) acquisitions are currently the workhorse for a variety of MR applications1-4. Recently, a low-cryogen, compact 3T (C3T) MRI system equipped with high-performance gradients (80 mT/m gradient amplitude and 700 T/m/s slew-rate, simultaneously) was developed and is under clinical evaluation in our institution5,6. The risk of peripheral nerve stimulation is substantially reduced5 on this system due to the smaller size of the gradient coil (42-cm inner diameter, 26-cm diameter-spherical-volume) even at its full gradient performance. The C3T system is capable of scanning brain, extremity, and infant. The 700 mT/m/s gradient slew-rate is 3.5 times higher than that of a conventional, 60-cm bore, whole-body gradient system such as GE MR750 (200 T/m/sec). The faster slew rate can significantly shorten the echo time for FSE/TSE. The reduced echo time can in turn improve signal level, shorten data acquisition time, and therefore improve image quality7. This system employs an asymmetric design for the transverse (x,y) gradient coils; the center of the imaging volume is shifted away from center of the coil towards the patient end, which facilitates patient access to imaging volume. However, this design gives rise to the concomitant field (CF) terms of the zeroth and first-order spatial dependences8, in addition to the second-order CF on the conventional whole-body gradients with symmetric design9. These additional CF can cause signal loss and image ghosting in FSE acquisitions10, potentially impairing the diagnostic value of FSE images within the clinically relevant spectra of acquisition parameters. We have previously reported real-time techniques to compensate for these additional CFs using gradient pre-emphasis11 and frequency shifting12. Here, the effect of these compensations on the image quality of FSE was evaluated based on a preliminary data set (n=11) subjects using a clinical, T2-weighted FSE protocol. We demonstrate that the CF compensation significantly improves quality of clinical FSE images acquired on the asymmetric gradient system.Methods
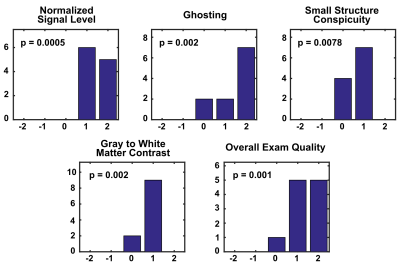
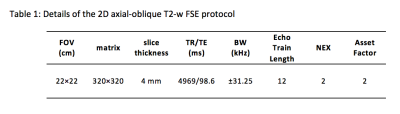
Under an IRB-approved protocol, eleven volunteers were scanned on the C3T system using a 32-channel (Nova Medical Inc., Wilmington, MA) (n=8) or 8-channel (Invivo, Gainesville, Florida) (n=3) receive-only brain coils and a 2D axial-oblique T2-weighted FSE acquisition (detailed in Table 1). Acquisitions were performed with and without the prospective zeroth/first-order CF compensations using real-time frequency shifting12 and gradient pre-emphasis11. These compensations were implemented on the RF and gradient subsystems responsible for B0 and linear gradient eddy-current compensation. The acquired images were evaluated in a blinded fashion by a board-certified neuroradiologist (26 years of experience) based on 1) relative signal level, 2) image ghosting, 3) small structure conspicuity, 4) white-to-gray matter contrast, and 5) overall exam quality. The image quality is graded on a five-point scale from -2 to +2, with -2 representing strong preference for images without CF compensation and +2 representing strong preference for images with CF compensation. The image quality under each criterion was compared using the one-sided (right-tailed) Wilcoxon signed rank tests (α=0.05) (null hypothesis: the images acquired without CF compensation perform better than, or equivalent to, the images with CF compensation).Results
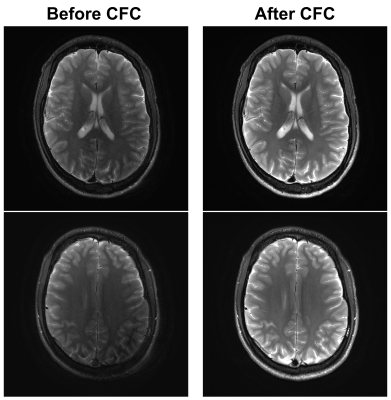
Figure 1 (left column) shows examples of images acquired without CF compensation which demonstrates noticeable loss of signal, image ghosting, and blurring of small structures such as the extravascular space. These effects are found to be more prominent towards the superior end of the brain (lower-left vs. upper-left). Performing real-time zeroth and first-order concomitant field compensations effectively reduces these artifacts (right column). Figure 2 summarizes the results of radiological evaluations of all five criteria. The right-sided Wilcoxon signed rank tests show that image quality in all categories is significantly improved after CF compensation (p<0.01 for all categories).Discussion
The C3T system with an high-performance gradient is a promising platform, which offers improved imaging performance for various applications including FSE/TSE. However, additional CFs due to the asymmetric gradient design can degrade the diagnostic quality of acquired images. Strong visual and statistical evidence support that the use of real-time CF compensation significantly improves the image quality by increasing signal level, reducing image ghosting, and improving small structure conspicuity and gray-to-white matter contrast. We focused on a clinical 2D T2-weighted FSE protocol in this comparison, since in our experience, the zeroth/first-order CF are not nearly as problematic for T1-weighted FSE/TSE acquisitions, due to their shorter TE time and less phase error accumulation along the echo train10.Conclusion
The compensation of the asymmetric gradient CF of the zeroth/first-order significantly improves image quality of a clinical FSE acquisition performed on an asymmetric, high-performance gradient system, and is therefore essential to enable the full capabilities of this type of system.Acknowledgements
This work was supported by research grant: NIH R01EB010065 and U01EB024450-01.
References
1. Hennig J, Nauerth A, Friedburg H. Rare imaging—a fast imaging method for clinical MR. Magn Reson Med 1986;3:823–833.
2. Hennig J. Multiecho imaging sequences with low refocusing flip angles. J Magn Reson 1988;78:397–407.
3. Jones KM, Mulkern RV, Mantello MT, Melki PS, Ahn SS, Barnes PD, Jolesz FA. Brain hemorrhage—evaluation with fast spin-echo and conventional dual spin-echo images. Radiology 1992;182:53–58.
4. Norbash AM, Glover GH, Enzmann DR. Intracerebral lesion contrast with spin-echo and fast spin-echo pulse sequences. Radiology 1992;185:661–665.
5. Lee SK, Mathieu JB, Graziani D, et al. Peripheral nerve stimulation characteristics of an asymmetric head-only gradient coil compatible with a high-channel-count receiver array. Magn Reson Med 2016;76:1939–1950.
6. Weavers PT, Shu Y, Tao S, Huston J III, Lee SK, Graziani D, Mathieu JB, Trzasko JD, Foo TK, Bernstein MA. Technical note: Compact three-tesla magnetic resonance imager with high-performance gradients passes ACR image quality and acoustic noise tests. Med Phys 2016;43:1259–1264.
7. Weavers PT, Campeau N, Shu Y, Tao S, Trzasko JD, Gray EM, Foo TKF, Bernstein MA, Huston J III. Improved T2-weighted 3D FLAIR from a compact, lightweight 3T scanner with high performance gradients. In Proceedings of the 25th Annual Meeting of the ISMRM, Honolulu, HI, 2017. p.4747.
8. Meier C, Zwanger M, Feiweier T, Porter D. Concomitant field terms for asymmetric gradient coils: consequences for diffusion, flow, and echo-planar imaging. Magn Reson Med 2008;60:128–134.
9. Bernstein MA, Zhou XJ, Polzin JA, King KF, Ganin A, Pelc NJ, Glover GH. Concomitant gradient terms in phase contrast MR: analysis and correction. Magn Reson Med 1998;39:300–308.
10. Tao S, Weavers PT, Trzasko JD, Huston J, Shu Y, Gray EM, Foo TKF, Bernstein MA. The effects of concomitant fields in fast spin echo acquisition on asymmetric MRI gradient systems. Magn Reson Med 2017. DOI 10.1002/mrm.26789.
11. Tao S, Weavers PT, Trzasko JD, Shu Y, Huston J III, Lee SK, Frigo LM, Bernstein MA. Gradient pre-emphasis to counteract first-order concomitant fields on asymmetric MRI gradient systems. Magn Reson Med 2017;77:2250–2262.
12. Weavers PT, Tao S, Trzasko JD, Frigo LM, Shu Y, Frick MA, Lee SK, Foo TKF, Bernstein MA. B0 concomitant field compensation for MRI systems employing asymmetric transverse gradient coils. Magn Reson Med 2017. doi:10.1002/mrm.26790.
Figures