Eliana Gianolio1, Enza Di Gregorio1, Giuseppe Ferrauto1, and Silvio Aime1
1Molecular Biotechnologies and Health Science, University of Torino, Torino, Italy
Synopsis
GBCAs are routinely used in many clinical MRI
protocols, their high stability should ensure that the Gd-complexes are
excreted intact without side effects. Recently, it was reported that tiny
amounts of Gd3+ may be retained in the brain of patients. The aim of
this study was to investigate, in an animal model, the in vivo fate of Gadoteridol
and Gadodiamide extending the investigation of Gd retention to other body
tissues besides brain. Several administration protocols differing for i) the
number of total doses, ii) the frequency of the administrations and iii) the
sacrifice time after the last administration, were compared.
Introduction
Recently, a renewed interest
on the possibility that Gadolinium Based Contrast Agents (GBCAs) may cause
adverse effects, came out because a number of studies reported the occurrence
of Gadolinium retention in the brain of patients that have been previously
administered with multiple doses of GBCAs.1
The aim of this study
was to use a validated animal model to compare different GBCAs i.v.
administration schemes in order to assess in which way they affect the accumulation
and distribution of Gd-containing species in the body tissues of healthy mice.Methods
Gadodiamide and
Gadoteridol have been compared. The accumulation of Gd in several
tissues/organs (cerebrum, cerebellum, spleen, liver, kidneys, eyes, skin, bone
and muscle) has been assessed by ICP-MS
upon administration of the GBCAs i) at different times after the last administration (3 weeks or 3 months),
ii) when one, three or twelve doses (0.1 mmol/Kg) of GBCA were administered and iii) when
administrations were made every two weeks.Results
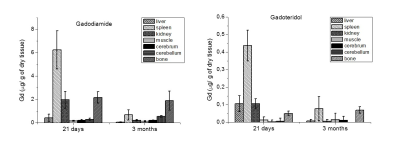
Gd was found in
all tissues after the administration of Gadodiamide. Conversely, in the case of
Gadoteridol, Gd was detected only in spleen, kidneys, liver and bone. For both
GBCAs the amount of Gd depends on the number of administered doses. The amounts
of Gd found in spleen, liver and kidneys markedly decrease upon increasing the
time that has passed after the last administration, whereas, in the case of
Gadodiamide, the decrease of Gd accumulated in bone, cerebrum and cerebellum appears
to occur at a much slower rate. Overall,
areas of long term deposition appear to be bone and spleen for both GBCAs.Discussion and Conclusion
The herein
reported results show that, in the case of Gadodiamide, significant amounts of Gd
are found in all the investigated organs, even when administered at low dose. Conversely,
in the case of Gadoteridol, Gd retention appears limited to some organs, only
upon repeated administrations.
In conclusion, our findings demonstrate that
intravenous multiple administrations of GBCAs is associated with extensive
multiorgan depositions which is reduced but not eliminated by the use of the
macrocyclic Gadoteridol as well as by adopting reduced and/or less frequent
dosing.Acknowledgements
The study was funded by
Progetto di Ateneo Compagnia di San
Paolo (CSTO160182). E.D.G and G.F. were supported by FIRC-AIRC (Fondazione
Italiana per la Ricerca sul Cancro AIRC) fellowships.References
Gulani V, Calamante
F, Shellock FG, et al. Gadolinium deposition in the brain: summary of evidence
and reccomendations Lancet Neurol. 2017;16:564‐570.