4195
Linear contrast agent-induced high signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images in pediatric patientsShintaro Ichikawa1, Utaroh Motosugi1, Yoshie Omiya1, and Hiroshi Onishi1
1Department of Radiology, University of Yamanashi, Chuo-shi, Japan
Synopsis
Hyperintensity in the dentate nucleus (DN) and globus pallidus (GP) on unenhanced T1-weighted images (T1WI) was associated with previous administration of linear gadolinium-based contrast agent (GBCA) in pediatric patients. The DN-to-pons ratio increased as the number of linear GBCAs administrations increased. The GP-to-thalamus ratio of the “Linear GBCAs ≥ 5 administrations” group was significantly higher than that of the “No GBCAs administration” and the “Linear GBCAs 1–4 administrations” groups. The number of previous administrations of linear GBCAs was positively correlated with the DN-to-pons ratio and the GP-to-thalamus ratio.
Purpose
Recent studies have reported an association between hyperintensity of the dentate nucleus (DN) and globus pallidus (GP) on T1-weighted images (T1WI) and a history of gadolinium-based contrast agent (GBCA) administration. Increased signal intensity of the DN on T1WI has been reported to be positively correlated with previous exposure to linear GBCAs, but not to macrocyclic GBCAs (1–3). Several reports have focused on pediatric patients (4–8). However, there is no report of the phenomenon in a Japanese population. Therefore, this study aimed to evaluate whether an association exists between a T1-signal increase of the DN or GP on T1WI and previous administration of linear GBCAs in pediatric patients.Methods
This retrospective study was approved by the institutional review board; the requirement for informed patient consent was waived. The inclusion criteria for participant enrollment were: (i) at least five consecutive administrations of linear GBCAs (gadodiamide or gadopentetate dimeglumine) followed by brain MRI, (ii) no history of administration of other or unknown GBCAs, and (iii) brain MRI using a 1.5-T scanner. A total of 42 patients were assigned to the “Linear GBCAs ≥ 5 administrations” group. Additionally, 42 patients with a history of 1–4 consecutive administrations of linear GBCAs and 42 with no history of GBCA administration (“Linear GBCAs 1–4 administrations” group and “No GBCAs administration” group) were included. Age and sex distributions were matched among the 3 groups. The final study included 126 patients (male:female, 72:54; median age, 16 (range 4–18) years) (Table 1).Results
The DN-to-pons ratio increased as the number of linear GBCAs administrations increased (P < 0.0001–0.0063, Fig. 1). The GP-to-thalamus ratio of the “Linear GBCAs ≥ 5 administrations” group was significantly higher than that of the “No GBCAs administration” and the “Linear GBCAs 1–4 administrations” groups (P < 0.0001, Fig. 1). The GP-to-thalamus ratio of the “Linear GBCAs 1–4 administrations” group did not differ significantly from that of the “No GBCAs administration” group (P = 1.0000, Fig. 1). The number of previous administrations of linear GBCAs was positively correlated with the DN-to-pons ratio and the GP-to-thalamus ratio (Fig. 2). The interobserver ICC for measurement of the DN-to-pons ratio was excellent (0.8236). That of the GP-to-thalamus ratio was good (0.6738). Figs. 3 and 4 present representative clinical cases. [Discussion] The results of this study indicate that hyperintensity of the DN and GP on T1WI is associated with previous administration of linear GBCAs in pediatric patients. These findings are consistent with previous reports (6–8). Flood et al. reported that a signal increase of the GP was not observed (6). Our study included patients with a history of gadodiamide administration, which is the least thermodynamically stable of the GBCAs. This may affect the discrepancy.Conclusion
High signal intensity of the DN and GP on T1WI is associated with previous administration of linear GBCAs in pediatric patients.Acknowledgements
No acknowledgement found.References
1) Kanda T, et al. Radiology. 2014;270:834-841. 2) Radbruch A, et al. Invest Radiol 2015;50:805-810. 3) Cao Y, et al. AJR Am J Roentgenol 2016;206:414-419. 4) Ramalho J, et al. J Magn Reson Imaging. Radiology 2015;276:836-844. 5) McDonald JS, et al. JAMA pediatr 2017;171:705-707. 6) Flood TF, et al. Radiology 2017;282:222-228. 7) Roberts DR, et al. AJNR Am J Neuroradiol 2016;37:2340-2347. 8) Hu HH, et al. Pediatr Radiol 2016;46:1590-1598.Figures
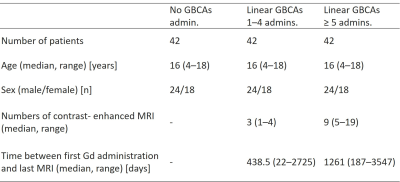
Table 1. Patient demographic data
Age and sex
distributions were matched among the 3 groups. The final study sample comprised
126 patients.
admin. =
administration, GBCA = gadolinium-based contrast agent.
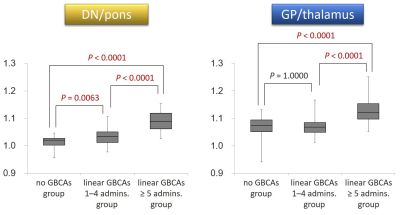
Fig. 1. Signal ratios of DN-to-pons and GP-to-thalamus
in the three groups.
The DN-to-pons
ratio increased as the number of linear GBCAs administrations increased. The
GP-to-thalamus ratio of the “Linear GBCAs ≥ 5 administrations” group was
significantly higher than that of the “No GBCAs administration” and the “Linear
GBCAs 1–4 administrations” groups. The GP-to-thalamus ratio of the “Linear
GBCAs 1–4 administrations” group did not differ significantly from that of the
“No GBCAs administration” group.
DN = dentate nucleus, GP = globus pallidus, GBCA = gadolinium-based
contrast agent, admin. = administration
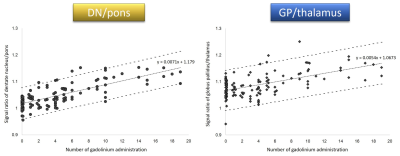
Fig. 2. Scatter plots of DN-to-pons and GP-to-thalamus
ratio and number of previous administrations of linear GBCAs.
The number of
previous administrations of linear GBCA was positively correlated with the
DN-to-pons ratio and the GP-to-thalamus ratio.
DN = dentate
nucleus, GP = globus
pallidus
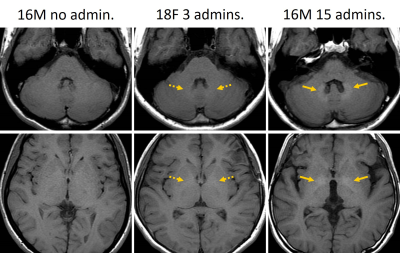
Fig. 3. Imaging findings of three cases with different
numbers of linear GBCAs administrations.
The signal
intensity of the DN increased as the number of linear GBCAs administrations
increased. However, there is no visual difference in the GP between patients
with histories of no and 3 linear GBCAs administrations. The signal intensity
of the GP of the patient with a history of 15 linear GBCAs administrations is
higher than that of the other patients.
DN = dentate nucleus, GP = globus pallidus, GBCA = gadolinium-based
contrast agent, admin. = administration
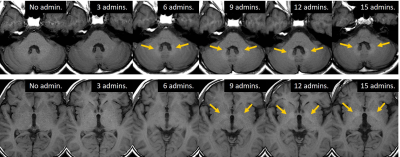
Fig. 4. Imaging findings of a temporal change in the
signal intensity of the DN and GP in the patient with 15 linear GBCAs
administrations.
The signal intensity of the DN increased earlier than
that of the GP as the number of linear GBCAs administrations increased.
DN = dentate nucleus, GP = globus pallidus, GBCA = gadolinium-based
contrast agent, admin. = administration