4182
Reducing PNS with Minimal Performance Penalties via Simple Pulse Sequence Modifications on a Compact 3T Scanner1Department of Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
More than 170 volunteers (patient subjects and normal controls) have been scanned since April 2016 following installation of a compact 3T MRI system with high-performance gradients (gradient strength=80mT/m, slew-rate=700T/m/s) at our institution. Despite peripheral nerve stimulation (PNS) related concerns associated with the high-performance gradients, no or minimal PNS sensation has been reported, with the exception of three normal control subjects. To mitigate the effect of PNS in these and other especially sensitive subjects, a simple pulse sequence modification was developed. Two of the subjects who reported PNS from their initial exam were available to be re-imaged with the modified pulse sequence, and subsequently reported no PNS.
Introduction
In MR imaging, one of the major concerns associated with high-performance gradients is the physiological effect of peripheral nerve stimulation (PNS). A recent study1 demonstrated that the occurrence of PNS could be substantially reduced on a compact 3T (C3T) even with high-performance gradients (gradient strength=80m T/m, slew-rate=700 T/m/s simultaneously) due to the reduced sized of the gradient coil compared to a whole-body scanner. Following installation of the C3T at our institution, more than 170 volunteers (patient subjects and normal controls) have been successfully scanned under an IRB-approved protocol since April 2016. PNS sensation was been reported in three subjects during a T2-weighted 3D-FSE FLAIR sequence. To reduce PNS in sensitive subjects, a simple pulse sequence modification with a minimal performance penalty was developed. Two of the three subjects were able to return for a repeat exam with the modified sequence on the C3T.Methods
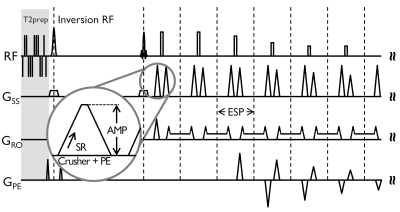
The original 3D-FLAIR sequence (designed for a 200 T/m/s system) includes strong and fast switching crusher gradients combined with slice-encoding lobes on the logical Z- (slice selection) axis (Fig. 1). A simple sequence modification was made to allow a user-specified degree of derating of slew-rate (SR) for the crusher lobe ramps, while leaving the total area under crusher gradient lobe unchanged. The echo spacing a function of the degree of slew rate derating was recorded.
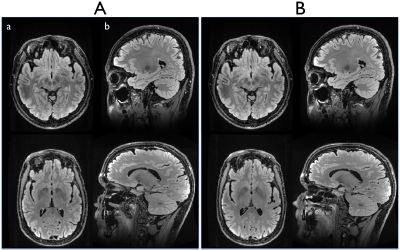
Two of the three subjects who previously experienced PNS were available to return for a repeat exam with the modified sequence on the C3T. They were re-imaged under an IRB-approved protocol. The PNS threshold level on both subjects was measured as function of the degree of slew rate derating. The repeated imaging used a 32-channel head coil (Nova Medical, Wilmington, MA) and concomitant field compensation2,3 for the asymmetric gradient design on the C3T. The imaging parameters were: TR/TE/TI=7600/94/2063 ms, in-plane and through-plane ASSET factor=2, readout bandwidth=±62.5 kHz, slice thickness=1.2 mm, echo train length=200, FOV=240×240 mm2, matrix size=256×256. The scan time was 6 minutes 46 seconds. The standard acquisition plane for the 3D-FLAIR is sagittal, but all three planes were acquired to test for PNS dependence. Finally, the reconstructed images from the modified sequence were compared by a board-certified neuroradiologist with those acquired in the previous (700 T/m/s crusher ramp) exam.
Results and Discussion
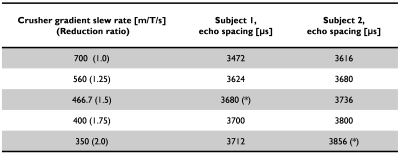
PNS was reported only in the sagittal acquisition, which corresponds to the crusher gradient waveforms being played on the physical X-axis (i.e., right-left). This is consistent with previous results where the physical-X gradient coil had lower PNS threshold compared to Y- and Z-coils1. As a function of SR for the crusher gradient, the measured PNS threshold levels from the two subjects were 466.7 and 350 T/m/s on the physical X-axis demonstrating some subject-dependence for the PNS threshold. As the SR decreased, the corresponding echo spacing increased accordingly, e.g. by a small amount on the order of few hundred microseconds (Table 1). Notably, the scan time remained unchanged even with the echo spacing penalty. In addition, no noticeable difference in image quality was observed with and without the proposed modification (Fig. 2). In a blinded comparison, equivalent image contrast and lesion conspicuity was reported.
We believe that the PNS was observed on the C3T before the pulse sequence modification because it was using “stock” software, originally designed for a whole-body system. The original pulse sequence designers had no reason to restrict the slew rate performance of the crusher ramp when using a standard 200 T/m/s system, so the ramp time was minimized. This caused PNS in 3/170 subjects with a sagittal 3D-FLAIR acquisition. Derating the gradient ramps for the problematic crusher gradient pulse resolved the problem, with negligible performance penalty.
Conclusion
The C3T routinely runs gradient performance of 80 mT/m and 700 T/m/s, simultaneously without PNS. Occasionally, PNS was observed (3 out of 170 subjects), with one specific pulse sequence acquisition (sagittal 3D FLAIR). Derating the entire system is not an optimal solution, because it causes performance penalties. Instead, we showed that the problematic gradient lobe can be pinpointed, and then selectively derated with a simple pulse sequence modification. That approach resolved the PNS issue with a minimal increase in echo spacing, and no effect on acquisition time or observed image quality.Acknowledgements
This work was supported by NIH U01 EB024450-01.References
1. Lee S-K, Mathieu J-B, Graziani D, et al. Peripheral nerve stimulation characteristics of an asymmetric head-only gradient coil compatible with a high-channel-count receiver array. Magn Reson Med 2016;76:1939–1950. doi: 10.1002/mrm.26044.
2. Tao S, Weavers PT, Trzasko JD, Shu Y, Huston J 3rd, Lee SK, Frigo LM, Bernstein MA. Gradient pre-emphasis to counteract first-order concomitant fields on asymmetric MRI gradient systems. Magn Reson Med. 2017 Jun;77(6):2250-2262. doi: 10.1002/mrm.26315.
3. Weavers PT, Tao S, Trzasko JD, Frigo LM, Shu Y, Frick MA, Lee SK, Foo TKF, Bernstein MA. B0 concomitant field compensation for MRI systems employing asymmetric transverse gradient coils. Magn Reson in Med 2017. doi:10.1002/mrm.26790
Figures