4172
Dedicated 3-Channel Surface Coil for Interventional Magnetic Resonance Imaging at 3T - First Evaluation1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Department of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 3Research Campus STIMULATE, Hannover, Germany, 4Siemens Healthcare USA, Baltimore, MD, United States, 5Siemens Healthcare USA, Malvern, PA, United States
Synopsis
We developed a dedicated interventional MRI coil with 3 receiver channels and a wide central aperture for interventional access, which we benchmarked against a 4-channel system surface coil. We assessed signal-to-noise-ratios (SNR), geometry (g)-factors and instrument artifact size in a phantom and 100 subjects. The interventional MRI prototype coil produced similar SNR values and g-factors than the 4-channel system coil. There was no significant difference in artifacts, which were suitable for accurate and safe procedures. The central aperture of the interventional MRI prototype coil improved access and the coil performed error-free during a large variety of interventional MRI procedures.
Introduction
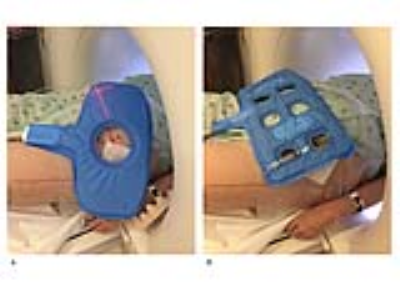
Interventional magnetic resonance imaging (MRI) at 3 Tesla is beneficial for a variety of procedures, due to its ability to visualize small anatomical structures, radiographically occult lesions, and monitor procedures such as cryoablations.1 Available system surface coils provide access, but are limited due to low playability, small apertures through which interventional instruments need to be inserted, and a high coil profile that may hinder shallow instrument angles. In order to gain sufficient access to and maintain sterility of the interventional site, a flexible surface coil with a low profile and wide central aperture is desirable.2 We developed a dedicated 3T interventional MRI coil with 3 receiver channels and a wide central aperture, which we benchmarked against a 4-channel system surface coil.Methods
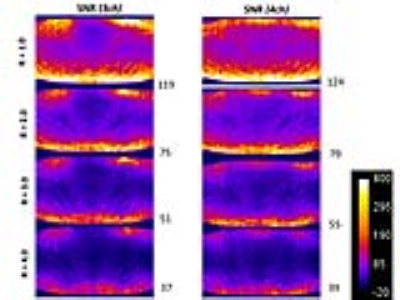
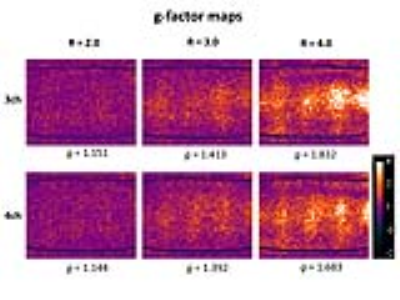
We obtained institutional review board approval and informed consent from all participating subjects. All experiments were performed on a commercially available wide-bore 3T MRI system. The interventional MRI prototype coil was designed by an expert panel of interventional MRI physicians and scientists and consisted of 3 receiver channels, a shallow profile, and a wide central aperture for interventional access. The interventional MRI prototype coil was benchmarked against an FDA-approved 4-channel system coil that had multiple small openings through which devices can be passed. Using an animal protein gel phantom, signal-to-noise-ratios3 (SNRs) and g-factors3 were determined with a maximum parallel imaging acceleration factor of 4. SNR was calculated as [average (SROI1, SROI2)]/[√2 *standard deviation(SROI1-SROI2)], where SROI represented the average signal on each matching region-of-interest (ROI) on two repeat images. G-factor was calculated as [SNRun-accelerated/(SNRaccelerated * √R)], where R represented the acceleration factor. The artifact size of a 20 gauge interventional needle was measured and compared at 4 different angulations relative to B0 using a high-bandwidth TSE pulse sequence. In 10 volunteers, SNR and CNR of various tissues were measured for various interventional TSE-based pulse sequences. Finally, the interventional MRI prototype coil was used in 100 subjects for a wide variety of interventional procedures.
Results
Both, the 3-channel interventional prototype and 4-channel system coils produced similar SNR value in the gel phantom, which decreased similarly with increasing parallel imaging acceleration factors (Figure 1). The SNR maps showed a similar signal distribution with the highest values adjacent to the receiver coils on top of the phantom and to the table coil elements at the bottom, whereas there was numerically slightly less signal along the central aperture. Both coils showed similarly increasing g-factors with increasing acceleration factors, whereas the g-factors were slightly higher for the interventional coil (Figure 2). There were no significant differences in the width and length of the artifact of the interventional needle for both coils regardless of needle angulation (Figure 3). In human subjects, there were no significant SNR/CNR differences of tissues with either coil (Figure 4). There was no visible loss of signal near the aperture of the interventional coil on interventional MR images. The interventional prototype coil improved access and performed error-free during a large variety of interventional MRI procedures (Figure 5).Discussion
Successful MR-guided interventions require accurate visualization of instruments and surrounding anatomy, but also sufficient access to the interventional site to ensure accurate instrument guidance and sterility. In addition, fast procedural image acquisition is important for short procedure times. Therefore, interventional coils should feature sufficient access through a larger enough central aperture, sufficient SNR and CNR, and parallel imaging capabilities. A previously described butterfly design showed promising results for interventional MRI at 1 Tesla.4 Our 3-channel interventional coil features a phased array design that permitted fast and accurate interventional MRI with SNR and CNR values that were comparable to a standard 4-channel surface coil at 3 Tesla. The noise distribution of the interventional coil was minimally more heterogeneous in the phantom evaluation with higher acceleration factors, which may be related to the central aperture. However, this characteristic was not visible on interventional MR images during the 100 procedures, suggesting it is of no practical importance.Conclusion
We developed a dedicated coil for interventional MRI at 3 Tesla that has similar image performance characteristics than a diagnostic 4-channel system surface coil. The customary design with wide central aperture provides improved access to and ensures sterility of the interventional site.Acknowledgements
We thank the members of the interventional MRI expert panel that participated in the design and realization of the interventional MRI coil.References
- Fritz J, Dellon AL, Williams EH, Belzberg AJ, Carrino JA. 3-Tesla High-Field Magnetic Resonance Neurography for Guiding Nerve Blocks and Its Role in Pain Management. Magn Reson Imaging Clin N Am. 2015 Nov;23(4):533-45
- Fritz J, Dellon AL, Williams EH, Rosson GD, Belzberg AJ, Eckhauser FE. Diagnostic Accuracy of Selective 3-T MR Neurography-guided Retroperitoneal Genitofemoral Nerve Blocks for the Diagnosis of Genitofemoral Neuralgia. Radiology. 2017 Oct;285(1):176-185.
- Reeder SB, Wintersperger BJ, Dietrich O, Lanz T, Greiser A, Reiser MF, Glazer GM, Schoenberg SO. Practical approaches to the evaluation of signal-to-noise ratio performance with parallel imaging: application with cardiac imaging and a 32-channel cardiac coil. Magn Reson Med. 2005 Sep;54(3):748-54.
- Rump JC, Streitparth F, Böning G, Seebauer CJ, Walter T, Güttler F, Hamm B, Teichgräber UK. Evaluation of a MR-quadrupole imaging coil for spinal interventions in a vertical 1.0 T MRI. Magn Reson Med. 2012 Aug;68(2):600-5. doi: 10.1002/mrm.23268. Epub 2011 Dec 27.
Figures