4163
Accuracy and Time Efficiency of Real-Time MRI-Guided Remote-Controlled Targeted Needle Placement During Motion Using Hydrostatic Actuators1Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 2Physics and Biology in Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 3Mechanical and Aerospace Engineering, University of California, Los Angeles, Los Angeles, CA, United States, 4Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 5Department of Radiology, Dongguk University Ilsan Hospital, Goyang, Korea, Democratic People's Republic of
Synopsis
In this work, we investigate the accuracy and time efficiency for real-time MRI-guided targeted needle placement using a rolling-diaphragm hydrostatic actuator system during motion. We show that the actuator-assisted approach was able to guide the needle to targets with greater accuracy and in less time than the conventional step-and-shoot strategy for both static and dynamic conditions using a programmable motion phantom. The new actuator system can potentially enable physicians to remotely perform real-time MRI-guided interventions during motion.
Introduction
MRI has unique advantages for guiding minimally invasive interventions(1–3), especially in the abdomen where motion is a major concern. However, the narrow gantry space of MRI scanners is a critical limitation, restricting physician's access to the patient(1,3,4). Previously, we demonstrated that a prototype master-slave rolling-diaphragm hydrostatic actuator system can be used to remotely guide a needle to targets under real-time MRI(5,6). In this work, we further evaluated the accuracy and time efficiency of human-operated remote-controlled targeted needle placement using the actuator system and compared it with respect to a conventional approach in both static and dynamic targets under MRI guidance.Methods
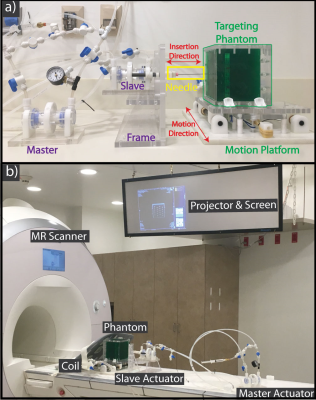
Motion Phantom Design: To evaluate the accuracy of needle placement using the actuator system, a phantom was designed to contain a targeting plate with twenty holes, each with a clinically relevant diameter of 10-mm(7). The phantom was filled with gelatin to obscure direct visualization (Fig. 1a). The phantom was secured to an in-house designed motion platform(8,9) to enable the evaluation of targeted needle placement without motion (static) and with reproducible sinusoidal motion of 0.3Hz and 20mm peak-to-peak amplitude (dynamic). The motion phantom was equipped with a function which allowed the operator to request a breath hold (20 sec) during all imaging and needle insertions.
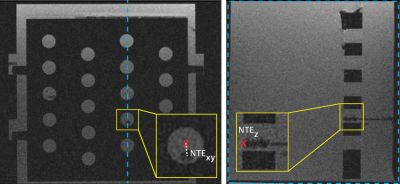
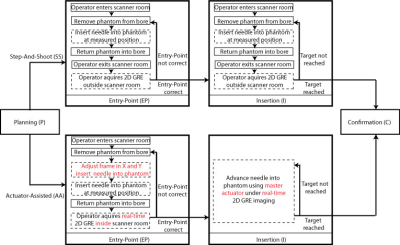
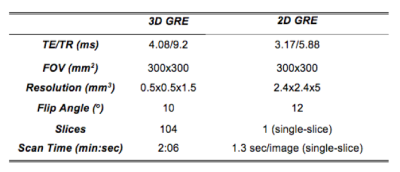
Targeted Needle Placement Under MRI Guidance: An operator was trained by interventional radiologists to consistently perform all experiments. The phantom was placed on the scanner table (Fig. 1b) and the operator was instructed to maneuver an MR-conditional needle (Invivo, 18G, 10cm) to a predefined position in a circular target (center in the X and Y directions, and far edge in the Z direction) (Fig. 2) during static (n=12) and dynamic (n=12) conditions. The novel real-time Actuator-Assisted (AA) method was compared to a conventional Step-and-Shoot (SS) method (Fig. 3). All cases began with a 3D GRE scan (Table 1) to locate the target (Planning). Next, the operator identified the point that would allow for a straight trajectory to the target (Entry-Point). For Insertion, the operator advanced the needle to the target using the prescribed targeting scheme (SS or AA). Finally, a set of 3D GRE scans were acquired for Confirmation.
Statistical Analysis: The needle-to-target error (NTE) and time for each procedural step were measured. Non-parametric tests were used to compare differences in the means (Mann-Whitney U test for static and Wilcoxon Sign Ranked test for dynamic targets) and group variance (Brown-Forsythe test) between the SS and AA methods for procedural times and NTE. Statistical significance was considered at the p<0.05 level.
Results
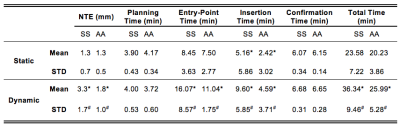
For the static targets, both the SS and the AA methods were able to achieve similar mean NTE (Table 2). Once the entry point was verified, AA significantly reduced the Insertion Time by 50% compared to SS. In dynamic targets, the AA method was significantly more accurate (lower mean NTE) and precise (lower standard deviation of NTE) than SS. The Insertion Time was again significantly reduced by 50% using AA in comparison to SS. In addition, the Entry-Point Time for AA was reduced by 30% and the Total Time was reduced by 25% versus SS.Discussion
Clinically, physicians require NTE<2.5 mm to target clinically relevant lesions with ≥5 mm diameter(10,11). Our results demonstrate that the proposed actuator system can attain this accuracy in both static and dynamic conditions using a reproducible motion phantom. For static targets, both the SS and AA methods achieved mean NTE<2.5 mm. Under dynamic conditions, the mean NTESS exceeded 2.5mm, while AA targeting was able to attain a significantly lower mean NTE<2 mm. The improved accuracy demonstrates that real-time visualization and AA remote control of needle insertion is advantageous in a dynamic setting, where motion can affect the needle and target positions.
Longer procedure times are a challenge for the clinical application of MRI-guided interventions. In static targets, the Insertion Time was significantly lower for the AA method compared to SS. Using the proposed AA method during dynamic conditions, the operator was able to visualize and anticipate when the target was aligned with the needle trajectory, leading to a significant reduction in both the mean and variation for the Entry-Point, Insertion, and Total time. These results demonstrate that the proposed system can potentially reduce procedure time to improve the application of MRI-guided interventions.
Conclusion
Using the proposed rolling-diaphragm hydrostatic actuator system, an operator was able to achieve targeted needle placement under real-time MRI guidance with significantly improved accuracy (mean NTE<2 mm) and reduced time (50% reduction for insertion) compared to a conventional step-and-shoot method in targets with motion. The proposed actuator system can potentially enable physicians to remotely perform real-time MRI-guided interventions even while the targets are in motion.Acknowledgements
No acknowledgement found.References
1. Kaye EA, Granlund KL, Morris EA, Maybody M, Solomon SB. Closed-Bore Interventional MRI: Percutaneous Biopsies and Ablations. Am. J. Roentgenol. [Internet] 2015;205:W400–W410. doi: 10.2214/AJR.15.14732.
2. Rothgang E, Gilson WD, Wacker F, Hornegger J, Lorenz CH, Weiss CR. Rapid freehand MR-guided percutaneous needle interventions: an image-based approach to improve workflow and feasibility. J. Magn. Reson. Imaging [Internet] 2013;37:1202–12. doi: 10.1002/jmri.23894.
3. Dupuy D, Fong Y, McMullen W, eds. Image-Guided Cancer Therapy: A Multidisciplinary Approach. Springer; 2013.
4. Kee ST, Murphy R MD ed. Clinical Interventional Oncology. Philadelphia: Elsevier
5. Mikaiel S, Simonelli J, Lee Y, Li X, Lee YS, Lu D, Sung K, Wu HH. Real-Time MRI-Guided Targeted Needle Placement During Motion using Rolling-Diaphragm Hydrostatic Actuators. In: ISMRM 25th Annual Meeting. ; 2017. p. 736. doi: 10.1002/jmri.21990.2.
6. Mikaiel S, Simonelli J, Lu D, Sung K, Tsao T-C, Wu HH. Real-Time MRI-Guided Interventions using Rolling-Diaphragm Hydrostatic Actuators. In: ISMRM 24th Annual Meeting. ; 2016. p. 3588.
7. De Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J. Hepatol. 2012;56:75–87. doi: 10.1016/S0168-8278(12)60009-9.
8. Simonelli J, Lee Y-H, Mikaiel S, Cheng-Wei C, Li X, Sung K, Lu D, Wu H, Tsao T-C. An MR-Compatible Stage for Respiratory Motion Emulation. In: 20th IFAC World Congress. ; 2017.
9. Mikaiel S, Simonelli J, Lee Y, Li X, Sung K, Tsao T, Wu HH. Hydrostatically Actuated MRI-Compatible Motion Platform for Dynamic MRI Research. In: ISMRM 25th Annual Meeting. Honolulu, Hawaii; 2017. p. 5557. doi: 10.1002/mrm.25903.2.
10. Kim YK, Kim YK, Park HJ, Park MJ, Lee WJ, Choi D. Noncontrast MRI with diffusion-weighted imaging as the sole imaging modality for detecting liver malignancy in patients with high risk for hepatocellular carcinoma. Magn. Reson. Imaging 2014;32:610–618. doi: 10.1016/j.mri.2013.12.021.
11. Rempp H, Clasen S, Pereira PL. Image-based monitoring of magnetic resonance-guided thermoablative therapies for liver tumors. Cardiovasc. Intervent. Radiol. 2012;35:1281–1294. doi: 10.1007/s00270-011-0227-6.
Figures