4159
Intraarterial chemotherapy of glioblastoma following osmotic blood-brain barrier opening under real-time MRI guidanceChengyan Chu1,2, Monica Pearl3, Yanrong Chen1,2, Anna Jablonska1,2, Xiaolei Song1,2, Miroslaw Janowski1,2, and Piotr Walczak1,2
1Russell H. Morgan Department of Radiology and Radiological Sciences, Division of MR Research, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Institute for Cell Engineering, Cellular Imaging Section, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Division of Interventional Neuroradiology, The Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Blood-brain barrier (BBB) prevents effective chemotherapy of brain tumors. Intra-arterial (IA) injection of hyperosmotic mannitol has been attempted for many years to permeabilize the BBB, however due to high variability, this procedure never became a routine clinical practice. We have previously shown that real-time MRI may circumvent that obstacle in large animal model. However, for drug screening purposes the mouse model if preferred. Here, we have shown that real-time, interventional MRI is also instrumental to precisely open BBB in rodents, and more importantly combining it with subsequent delivery of chemotherapeutic drug melphalan provides therapeutic benefit warranting consideration of clinical application.
Purpose
To develop safe and reproducible technique to disrupt local BBB in mice through guidance of interventional MRI and to improve efficacy of IA chemotherapy of brain tumors including glioblastoma.Methods
Naïve or human glioblastoma inoculated scid mice (20-25g, Jackson Laboratory) were anesthetized with 2% isoflurane gas. Briefly, the occipital artery was cauterized, the ECA and pterygopalatine arteries (PPA) were ligated temporarily with 4-0 silk sutures. The common carotid artery (CCA) was catheterized with a microcatheter. The mice with intra-arterial (IA) catheter secured were positioned in a Bruker 11.7T MRI scanner. Baseline T2 (TR/TE 2500/30) and T1 (TR/TE 100/1.88) weighted and dynamic GE-EPI (TE=3.0ms, TR=60ms, FOV=18, matrix=128, and acquisition time=92s and 16 repetitions) images of the brain were acquired. IA Feridex (dissolved in saline at 1:100; 0.1mg Fe/ml) was infused under dynamic GE-EPI to predict perfusion territory at specific speeds. 25% mannitol was delivered via IA route over one minute at the speed determined by previous Feridex injection. MRI (T1, T2-weighted) and histology were used to assess status of the blood brain barrier (BBB) and any consequences compromising safety of the procedure. For the glioblastoma-bearing mice, the BBB opening was followed by subsequent IA melphalan infusion (0.05mg/mouse) as a one-stop-shop.Results
We have previously reported a strong association between IA infusion speed and perfusion territory in large animals1. Here, we observed similar dependence for mice and real-time MRI was essential for adjusting the injection speed for safe and predictable brain targeting. Initially our transcatheter SPIO contrast delivery, at the infusion rate between 0.0008-0.0016ml/s resulted in inconsistent cerebral perfusion. However, when the speed was increased to the level between 0.0015 and 0.0025 ml/s, desired brain perfusion was obtained as visualized by characteristic reduction in signal intensity for the duration of injection bolus (Fig.1a-b). Gadolinium-enhanced T1 weighted scan showed hyperintensity in the region previously highlighted by the contrast infusion at the same rate (Fig.1c). This indicates successful BBB opening by IA mannitol as predicted by perfusion pre-scan. In histopathological validation using Evans Blue, which is a gold standard for BBB assessment and rhodamine, which was used a surrogate marker of therapeutic agent demonstrated pattern of extravasation that was consistent with the observed by MRI (Fig.1d). There was strong correlation of the SPIO perfusion pre-scan and T1+Gd enhancement (Fig.1e). In assessment of safety of the BBBO procedure T2-weighted MRI 3 days post BBBO showed no obvious abnormalities and the T1+Gd enhancement was not observed any more(Fig.2a). Immunohistochemistry for GFAP and IBA-1 7 days post BBBO showed no activation of astrocytes or microglia (Fig.2b-c). Similarly, there was no evidence of neuronal damage based on NeuN staining (Fig.2d). Altogether these data indicate excellent safety profile of BBB opening procedure. Subsequent experiments were performed in mice implanted with luciferase-expressing glioblastoma (Fig.3a). Tumor bearing mice were subjected to BBB disruption under interventional MRI (Fig.3b) followed by 0.05mg IA melphalan treatment. Longitudinal bioluminescent imaging revealed marked reduction of signal intensity six days after IA chemotherapy compared to no chemotherapy controls indicating treatment response. However, after this initial reduction the signal in treated mice continued to grow.Discussion
The goal of BBBO is to maximize CNS targeting of the therapeutic agent with minimizing systemic toxicity. However, osmotic BBBO proved highly variable and inconsistent2. This is also reflected by inconsistent methodology of preclinical studies. The infusion velocity of mannitol into carotid artery varied particularly, and in many animal studies extremely exceeded physiological rate of brain perfusion and had to be damaging3-4. Our MRI-guided IA injection experiments in several species show the value and importance of imaging allowing adjustment of infusion rate and open BBBO at physiologically relevant non-damaging infusion rates5-6. Our work showed that IA melphalan following BBBO can reach and affect the tumor growth. Transient nature of this effect is likely due to chemoresistance. In the future studies, we will potentiate the anti-tumor effect by adjusting parameters, for example, higher drug concentration, longer duration of mannitol infusion and will administer different chemotherapeutic agents with the same targeted approach.Conclusions
MRI guidance enables precise fine-tuning of the infusion rate to achieve safe and effective local BBBO at a high reproducibility. Bypassing the BBB obstacle resulted in treatment response, but the effect was transient.Acknowledgements
Supported by NIH/NINDS R01NS091110References
1. Janowski, M.; Walczak, P.; Pearl, M. S., Predicting and optimizing the territory of blood–brain barrier opening by superselective intra-arterial cerebral infusion under dynamic susceptibility contrast MRI guidance. Journal of Cerebral Blood Flow & Metabolism 2016, 36 (3), 569-575. 2. Joshi, S.; Ergin, A.; Wang, M.; Reif, R.; Zhang, J.; Bruce, J. N.; Bigio, I. J., Inconsistent blood brain barrier disruption by intraarterial mannitol in rabbits: implications for chemotherapy. J Neurooncol 2011, 104 (1), 11-9. 3. Foley, C. P.; Rubin, D. G.; Santillan, A.; Sondhi, D.; Dyke, J. P.; Crystal, R. G.; Gobin, Y. P.; Ballon, D. J., Intra-arterial delivery of AAV vectors to the mouse brain after mannitol mediated blood brain barrier disruption. J Control Release 2014, 196, 71-78. 4. Yang, W. L.; Barth, R. F.; Leveille, R.; Adams, D. M.; Ciesielski, M.; Fenstermaker, R. A.; Capala, J., Evaluation of systemically administered radiolabeled epidermal growth factor as a brain tumor targeting agent. Journal of Neuro-Oncology 2001, 55 (1), 19-28. 5. Walczak, P.; Wojtkiewicz, J.; Nowakowski, A.; Habich, A.; Holak, P.; Xu, J.; Adamiak, Z.; Chehade, M.; Pearl, M. S.; Gailloud, P.; Lukomska, B.; Maksymowicz, W.; Bulte, J. W.; Janowski, M., Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. J Cereb Blood Flow Metab 2016. 6. Janowski, M.; Lyczek, A.; Engels, C.; Xu, J.; Lukomska, B.; Bulte, J. W.; Walczak, P., Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cereb Blood Flow Metab 2013, 33 (6), 921-7.Figures
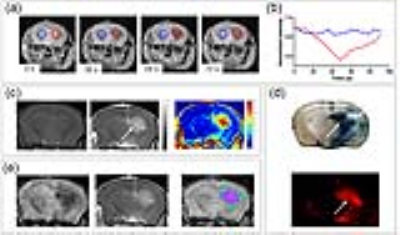
Figure 1. Use of real-time MRI to predict perfusion territory and
assessment for BBBO.
(a, b) The 0.025 ml/s rate
of infusion results in efficient cerebral perfusion, as marked by decrease in
pixel intensity (red circle = ROI, quantified in b). Signal intensity is
normalized by setting the value of ROIs as 1. (c) Pre- and 10 minutes post-Gd,
T1-weighted images show the signal enhancement in the treated hemisphere.
Color-coded subtraction image highlights the difference in signal intensity.
(d) Evans Blue and Rhodamine extravasation following mannitol treatment. (e) The
area of trans-catheter perfusion was overlaid on the Gd enhancement with
visualized on color-coded subtraction image.
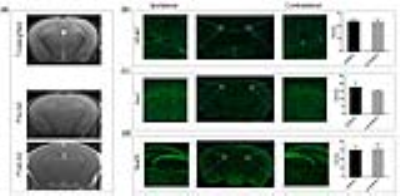
Figure 2. MRI imaging and histological appearance assessed for brain
damage post BBBO. (a) T2-weighted MRI with no evidence of
delayed brain damage 3 days post BBBO. The GFAP-, Iba1- and NueN-positive cells
are quantified by the intensity expressed as means ± SD, compared to untreated
hemisphere and differences are not significant.
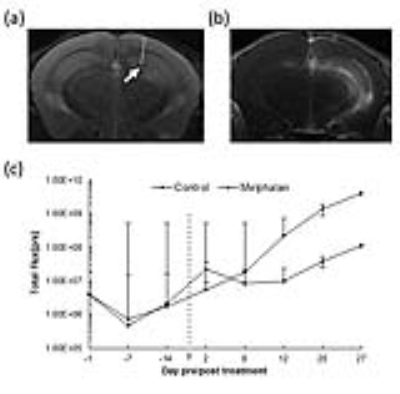
Figure 3. Effect of IA melphalan on glioblastoma following mannitol
induced-BBBO.
(a) T2-weighted MRI
localized the implanted tumor (arrow). (b) Gd enhancement was detected in the
region of the tumor (c) Bioluminescence monitoring of tumor growth pre and post
Melphalan treatment.