4154
Noninvasive assessment of great vessel stents using a susceptibility-based imaging method1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, shenzhen, China, 2Texas A&M University, Texas, TX, United States, 3Guangzhou Medical University, Qingyuan, China, 4Guangzhou Medical University, Guangzhou, China
Synopsis
Previous studies have demonstrated that a susceptibility-based positive contrast MR method exhibits excellent efficacy for visualizing MR compatible metal devices by taking advantage of their high magnetic susceptibility. However, the method was not evaluated in the assessment of stent restenosis. The purpose of this study is to assess whether the susceptibility-based positive method can be used to assess the stent restenosis, with the comparison of two typical MR positive contrast techniques, i.e., SUMO and GRASP. The experimental results showed that the susceptibility-based method not only provides better localization of the stent than SUMO and GRASP but also has capabiltiy to assess the stent restenosis.
Introduction
MR-compatible metallic stents have been widely used for the treatment of arterial occlusive disease1. However, conventional MR techniques have difficulty to access the stent restenosis because of the effects of susceptibility and radiofrequency (RF) shielding artifacts caused by stent graft. Previous studies have demonstrated that a susceptibility-based positive contrast method exhibits excellent efficacy for visualizing MR compatible metal devices by taking advantage of their high magnetic susceptibility2,3. However, it is not evaluated in the assessment of stent restenosis. Therefore, the purpose of this study is to prospectively assess whether the method can be used to assess the stent restenosis, with the comparison of two typical MR positive contrast techniques, i.e., SUMO and GRASP4,5. Experiment results showed that the susceptibility-based method not only provides better localization of the stent but also has capability to assess the stent restenosis.Theory
Susceptibility-based positive contrast method: This method employs a modified 2D Fast spin echo (FSE) sequence to accelerate data acquisition3. Two datasets with and without readouts shifted are acquired for measuring the field induced by the stent. Thus, the amount of phase change induced by the local susceptibility difference between the stent and the surrounding tissues is accumulated during Tshift. After the data acquisition, a kernel deconvolution algorithm is used to calculate the susceptibility mapping and thus the positive contrast image of stent is realized.
SUMO: This method considers the local susceptibility gradient as an additional gradient which leads to an echo-shift in k-space for the signal affected by the susceptibility. Thus, a modified k-space filter can be applied into k-space to get the echo-shift and a positive-contrast image is then generated by a map of the strength of the susceptibility-gradient vector.
GRASP: This method can directly obtain the positive contrast image by a modified gradient echo sequence. As the signal in the region near the nitinol stent is conserved because the induced dipole field compensated for the dephasing gradient, a hyperintense signal is observed near the nitinol stent against the dark background.
Methods
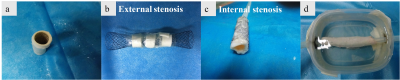
Experiment: A Ni-Ti tracheal stent was implanted into a portion of thoracic pig aorta which was used to simulate the stent restenosis, i.e., no stenosis (Figure 1a) by using the approach introduced by Nordmeyer J6, The external stenosis was formed by wrapping a plastic tie around the stent implantation site (Figure 1b), and external stenosis was fashioned by suturing aortic vessel material into the inner surface of the stent (Figure 1c). The aorta model was housed in a methylcellulose filled plastic container (Figure 1d) after the making of the stent phantom and attached to a peristaltic pump. Then the stent restenosis phantom was imaged with the three methods using the same 3T MR scanner. In the susceptibility-based method, Tshift is 0.6 ms ,TR/TE= 2000 /18 ms, resolution =0.67×0.67 mm2, slice thickness = 1.5 mm. For the SUMO, a 3D GRE sequence was used,TR/TE= 19/2.5 ms, resolution=1.04×1.04×2.8 mm3. For the GRASP, TR/TE= 3000 /5 ms, resolution=0.9×0.9×4 mm3, the gradient rephasing was respectively set to 40%, 60%, and 30% (no stenosis, external stenosis, and internal stenosis) of that used in the ordinary 2D GRE sequence.
Image analysis: Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated by comparing the reviewers' assessment of the stent. Then, data from the several reviewers was combined for further analysis.
Results
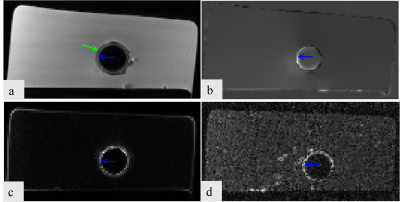
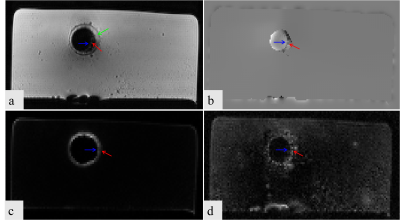
All the three methods successfully realized the positive contrast imaging of the stent (Figure 2&4). The green arrow indicated the position of the vessel wall of the pig aorta, the blue arrow indicated the position of the stent, and the red arrow indicated the restenosis. The susceptibility-based method provides excellent delineation of the stent lumen and good visualization of the stenosis compared to SUMO (Figure 3c & 4c) and GRASP (Figure 3d & 4d). It has higher sensitivity, specificity, NPV, and PPV whether the external stenosis or internal stenosis. GRASP performs poorly in the visualization of the internal stenosis (Figure 4d), but good assessment in the external stenosis (Figure 3d). However,the SUMO is the worst in assessing the stent restenosis. Besides, GRASP and SUMO broaden the stent lumen which results in the misrepresentation of the location of the stenosis. These observations show that the proposed method has the potentiality in imaging of great vessel stents and assessing the stent restenosis.Conclusion
Compared to SUMO and GRASP, the susceptibility-based method provides more accurate localization of the stent and shows better performance in the assessment of stent restenosis. As the proposed susceptibility-based method can accurately localize the position of the MR compatible metal devices and be used to access the stent restenosis, it may have potential to be used in the interventional MR clinical application.Acknowledgements
This work was supported in part by the National Science Foundation of China (81729003, 81571669, 61201442, 81501463, 61471350),National Science Foundation of USA (1606136), the National Key Research and Development Program of China (2016YFC0100302), and the Natural Science Foundation of Shenzhen (JCYJ20160531174850658, GJHZ20150316143320494, JCYJ20140417113430603, KQCX2015033117354154), the Excellent Youth Scholars of SIAT (Y5G003), and the Science and Technology Program of Guangdong (2015A020214019)References
[1] Lammer J, Bosiers M, Zeller T, Schillinger M, et al.First clinical trial of nitinol self-expanding everolimus-eluting stent implantation for peripheral arterial occlusive disease.Journal of Vascular Surgery, 2011, 54(2): 394-401.
[2] Dong Y, Chang Z, Xie G, Whitehead G, Ji JX. Susceptibility-based positive contrast MRI of brachytherapy seeds. Magn Reson Med, 2014, 74:716-726.
[3] Shi C, Xie G, Zhang Y, et al. Accelerated susceptibility-based positive contrast imaging of MR compatible metallic devices based on modified fast spin echo sequences [J]. Physics in Medicine and Biology, 2017, 62(7): 2505.
[4] Varma G, Clough R E, Acher P, Senegas J, Dahnke H, Keevil S F and Schaeffter T. Positive visualization of implanted devices with susceptibility gradient mapping using the original resolution. Magn Reson Med, 2011, 65: 1483-1490.
[5] Mani V, Briley-Saebo K C, Itskovich V V, Samber D D and Fayad Z A. Gradient echo acquisition for superparamagnetic particles with positive contrast (GRASP): sequence characterization in membrane and glass superparamagnetic iron oxide phantoms at 1.5T and 3T. Magn Reson Med, 2006, 55:126-35.
[6] Nordmeyer J, Gaudin R, Tann O R, et al. MRI may be sufficient for noninvasive assessment of great vessel stents: an in vitro comparison of MRI, CT, and conventional angiography[J]. American Journal of Roentgenology, 2010, 195(4): 865-871.
Figures