4153
Active-tracked versus Passive-tracked MRI-guided Cervical-cancer Brachytherapy catheter placement in 11 patients: Improved accuracy and reduced procedure time1Radiology, Brigham and Women's Hospital, Boston, MA, United States, 2Biomedical Engineering, King's College London, London, United Kingdom, 3Cardiology, Johns Hopkins University, Baltimore, MD, United States, 4MRI Interventions Inc., Irvine, CA, United States, 5Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 6Siemens Healthcare Inc., Boston, MA, United States, 7Radiation Oncology, Brigham and Women's Hospital, Boston, MA, United States, 8Radiation Oncology, Johns Hopkins University, Baltimore, MD, United States
Synopsis
We performed MRI-guided placement of high dose rate (HDR) brachytherapy catheters in 11 cervical-cancer patients within a 3.0 T MRI scanner. We compared placing MR-tracked metallic stylets to passively-tracked conventional stylets. Comparisons were performed during three procedure stages: coarse stylet navigation to the approximate region of the tumor; fine-tuned navigation to the clinician’s desired (final) location; stylet pull-back (withdrawal) from the body, which provided catheter trajectories for Radiation Treatment Planning. Active-tracking’s main benefits; (I) catheters placed much closer to the clinician’s intended location, including via complex manipulations requiring complete withdrawal and repositioning, (II) placement durations similar to transrectal-ultrasound guided HDR procedures.
Introduction
MR imaging is widely used in Radiation Therapy (RT) due to its superior delineation of the target volume, and the surrounding organs at risk (OARs). In High Dose Rate (HDR) brachytherapy, 12-30 interstitial catheters are directly placed into the tumor in order to deliver spatially-focused (<5mm diameter), high radiation dose to the critical target volume (CTV), while sparing OARs. MRI-guided brachytherapy is currently performed at a few sites using metallic stylets (rigid rods placed inside catheters in order to advance catheters through soft tissue) that are inserted with passively-tracked monitoring1. However, MRI image-based catheter identification is time-consuming (120-180 s/catheter-location-change), and insufficiently (~3x3x3mm3) accurate, requiring a CT scan after placement is completed to accurately identify the catheters’ spatial trajectories for radiation treatment planning (RTP), so MRI placement is not widely performed.
We developed dedicated MR-tracking (MRT) software for use with metallic devices, resolving B1 issues associated with multiple neighboring metallic structures. To meet RT requirements, with the navigational FOV >25x25x25 cm3, MRT gradient-distortion correction was applied. We also developed a commercial-grade active metallic stylet (MRI interventions, Irvine, CA) that included two multilayer printed-circuit micro-coils at its distal end, designed specifically to reduce B0-gradient effects close to metal surfaces, allowing MRT of the stylet tip’s location and orientation. Patient isolation circuitry was added to enable human use. We used the active stylets initially to place catheters into cervical cancers in 3 patients2. We demonstrated thereafter that the accuracy of MRT stylet trajectories, acquired during stylet pullbacks, was equivalent to CT3, removing the need for CT. We then showed4 that real-time tracking allowed for adaptive radiation treatment planning, varying the treatment plan during catheter placement, which may improve target dose coverage.
In the current IRB-approved study, we examined in 11 cervical-cancer patients whether (a) MRT placement improved CTV targeting, and (b) whether it improved the temporal efficiency of MRI-guided HDR brachytherapy, relative to passive MRI-guided placement.
Methods
Procedure: In each patient, based on pre-operative MRI scans, 12-30 catheters with conventional (non-active) stylets were inserted in a 3.0 T MRI using passive tracking, utilizing T2-weighted Turbo Spin Echo (T2-w) scans in 3 orthogonal directions (180 s/catheter-location-change). Passive navigation was concluded when the practicing clinician was satisfied with placement (mean time: 30 minutes). These final locations were designated baseline locations for subsequent active manipulation. MRT stylets were substituted in 2-6 catheters/patient, and active catheter navigation (6-16 frames/second, 0.6x0.6x0.6 mm3 resolution) was performed by a clinician working inside the MRI bore, using monitor feedback. Catheter tip location and orientation were computed and overlaid on T2-w “roadmap” images displayed on in-room monitors. Detailed imaging and tracking parameters are available5.
Three modes of actively tracked navigation were analyzed: (I) coarse navigation to the approximate region inside or around the tumor; (II) fine-tuned navigation, bringing the stylets to the desired location; and (III) pullback, with MRTR stylets rapidly withdrawn from within the catheters, providing catheter trajectories for RTP. MRT catheter 3D (x, y, z) micro-coil and tip locations as a function of time were continuously recorded.
Data Analysis: After catheter insertion, the clinician segmented tumor and OAR boundaries, which were overlaid on T2-w images using 3D Slicer RT6. MRT data was analyzed with MATLAB, utilizing the 3D position of each catheter’s tip during periods of active manipulation. We examined (A) the total three-dimensional Root-Mean-Square displacement between the first (baseline)and last time-point locations, (B) the average velocity of motion and (C) the average spacing between tracking time-points during stages I-III.
Results
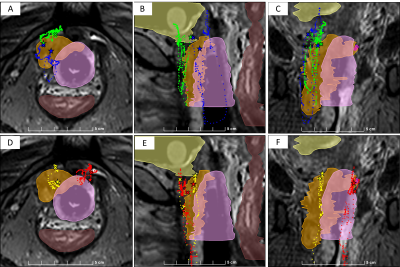
An exemplary time-history of MRT motion is shown in Fig. 1. In many cases, due to catheter deflection by soft-tissue resistance, improving positioning required complete withdrawal and re-insertion of the catheters, which was difficult to perform with passive tracking alone. Coarse and fine-tuned MRT repositioning resulted in a mean positional improvement, relative to passive tracking, of 16 and 28 mm, respectively. Mean speeds of coarse and fine-tuned repositioning were 33.4 and 11.6 mm/s, respectively, requiring mean procedural durations of 14 and 24 s, respectively. Pullbacks required a mean of 4.7 s, at a mean speed of 52 mm/s, and provided the complete catheter trajectory at 2 mm increments. All MR-tracked procedures were completed in <15 minutes.Conclusions
Active MR-Tracking resulted in a significant improvement in positioning, sometimes bringing a catheter from outside the tumor into its center. Speeds of the MRT-based procedure were similar to those observed with TRUS-based7 HDR procedures, surpassing the maximal 0.5 Hz/image speed possible, for equivalent resolution, with passively-tracked MRI. MRT pullback obviated digitizing locations from CT or MRI images, which can require hours of work. Assessment of reduced dose to OAR due to MRT positioning will require a longer follow-up time.Acknowledgements
NIH P41EB015898, NIH R21CA167800, NIH R01EB020667References
1. Viswanathan AN, Cormack RA, Holloway CL, et al. Magnetic resonance-guided interstitial therapy for vaginal recurrence of endometrial cancer. Int J Radiat Oncol Biol Phys 2006; 66: 91-99.
2. Wang W, Dumoulin CL, Viswanathan AN, et al. Real-time active MR tracking of metallic stylets in MR-guided radiation therapy. Magn Reson Med 2015; 73: 1803-1811.
3. Wang W, Viswanathan AN, Damato AL, et al. Evaluation of an active magnetic resonance tracking system for interstitial brachytherapy. Med Phys 2015; 42: 7114-7121.
4. Wang W, Viswanathan AN, Damato AL, et al. Integration of Active MR Tracking into Adaptive Radiation Therapy Treatment Planning. Proc Intl Soc Mag Reson Med 2015; 3819
5. De Arcos J, Schmidt EJ, Wang W, et al, Prospective Clinical Implementation of a Novel Magnetic Resonance Tracking Device for Real-Time Brachytherapy Catheter Positioning. Int J. Radiat Oncol Biol Phys 2017; 99: 618-626
6. Printer C, Lasso A, Wang A, et al. SlicerRT: Radiation therapy research toolkit for 3D Slicer, Med. Phys. 2012; 39: 6332-6338
7. Sharma DN, Rath GK, Thulkar S, et al. Use of transrectal ultrasound for high dose rate interstitial brachytherapy for patients of carcinoma of uterine cervix. J. Gynecol Oncol 2010; 21:12-17.
Figures