4145
Simultaneous Multi-slice Ultra-short Echo Time Imaging Using POMP Half Pulses and CAIPI1JABSOM, University of Hawai'i, Honolulu, HI, United States, 2University of Hawai'i, Honolulu, HI, United States
Synopsis
Phase offset multi-planar (POMP) encoding is applied in a simultaneous multi-slice (NSMS = 4) excitation scheme using half sinc pulses. In combination with a gradient echo 2D spiral readout trajectory and controlled aliasing in parallel imaging (CAIPI) via a model-based reconstruction a fast ultra-short echo time (UTE) sequence is developed. Sequence details and performance tests on short T2* phantoms are presented.
Introduction
UTE imaging enables the visualization of short T2* tissue like ligament, white matter or cortical bone that is not detectable with conventional MRI techniques. (1) In diseases such as Alzheimer’s or multiple sclerosis crucial information on the pathogenesis may be obtained from imaging these types of tissues. (2) Often half pulse slice-selective excitation is used with a center-out readout to obtain a 2D acquisition with a short TE. (3) However, challenges related to the fast signal decay due to the short transversal relaxation time (T2) of the tissue of interest make it difficult to obtain images of sufficient quality for clinical application in a reasonable time frame. Furthermore the use of half pulse excitation requires the summation of two pulses with opposite gradient polarity for slice selection. In this work we propose to decrease the acquisition time of the UTE half pulse method using simultaneous multi-slice (SMS) methods. While SMS imaging has found widespread application in combination with conventional slice-selective sinc pulses, UTE imaging using half pulses has not benefited from it so far partially due to the need of phase encoding gradients for optimal CAIPI sampling. Here we describe a UTE SMS approach using POMP encoding in combination with a CAIPI under-sampling pattern using spiral trajectories that are compatible with the requirements of short TEs in UTE sequences.(4,5)Methods
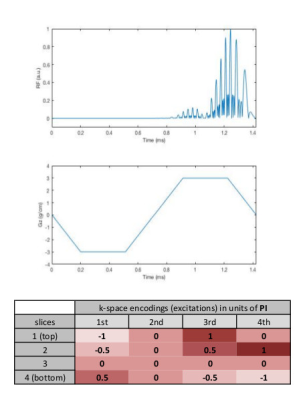
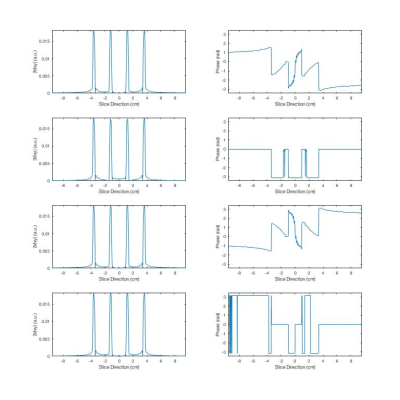
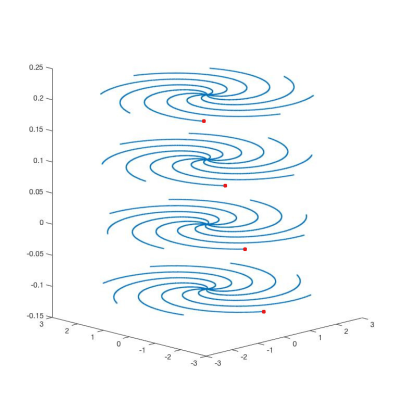
NSMS = 4 SMS half pulses were designed using the small tip angle approximation. Each individual slice was given a different phase to obtain the phase pattern corresponding to one plane in kz-space according to the POMP encoding. A total of four pairs of different half pulses were designed. Figure 1 shows the pulses including the excitation profiles including the phase encoding scheme obtained using Block equation simulations in Matlab (Mathworks, Natick, MA). MRI measurements were performed on a Siemens MAGNETON Prisma 3T (Erlangen, Germany) scanner equipped with a 52-channel head coil. Data were acquired using a gradient echo (NEcho=2) 2D spiral trajectory (Ntot=32) over 22 cm FOV with a 128x128 matrix size, TE of 30 μs and 2.2 ms, TR of 400 ms and gradient spoiling. The final images were obtained using a generalized reconstruction method. The spiral acquisition was retrospectively undersampled such that each pair of half pulses used a subset of the interleaves forming a rotated spiral for an R=1, 2 and 4 CAIPI style acquisition. The k-space trajectories and data were inserted directly into the signal equation and solved numerically using a non-uniform Fast Fourier Transform within a regularized conjugate gradient algorithm.(6,7) No coil sensitivity information was used for acceleration. All reconstructions were performed offline using Matlab. The sequence was tested on a phantom containing differently concentrated aqueous MnCl2 solutions (up to 200 mM).Results
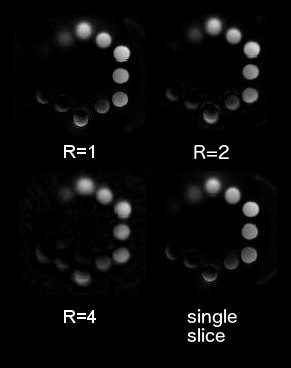
The POMP UTE sequence was tested on a short T2 phantom and compared to a corresponding single slice UTE sequence (figure 4). During the iterative reconstruction the simultaneously excited slices were quickly separated and no significant slice-cross-talk could be observed after 20 iterations. The fully sampled images (R=1) are comparable to those of the single slice acquisition. Even with an undersampling factor of R=2 no significant loss of image quality occurs, however, with R=4 artifacts become more pronounced.Conclusion
In conclusion the use of POMP and SMS provides a potential means for increasing speed for half pulse UTE acquisitions. In this example no coil sensitivity information was used and good slice separation was still observed. Further accelerations may be obtained using parallel imaging approaches.Acknowledgements
This project was supported by the NIH grants R01DA019912, R21EB02076, and K02DA020569.References
1. Wilhelm, M. J., Ong, H. H., Wehrli, S. L., Li, C., Tsai, P.-H., Hackney, D. B., & Wehrli, F. W. (2012). Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proceedings of the National Academy of Sciences of the United States of America, 109(24), 9605–9610.
2. Laule, C., Vavasour, I. M., Kolind, S. H., Li, D. K. B., Traboulsee, T. L., Moore, G. R. W., & MacKay, A. L. (2007). Magnetic resonance imaging of myelin. Neurotherapeutics : the Journal of the American Society for Experimental NeuroTherapeutics, 4(3), 460–484.
3. Sheth, V., Shao, H., Chen, J., Vandenberg, S., Corey-Bloom, J., Bydder, G. M., & Du, J. (2016). Magnetic resonance imaging of myelin using ultrashort Echo time (UTE) pulse sequences: Phantom, specimen, volunteer and multiple sclerosis patient studies. NeuroImage, 136(C), 37–44.
4. Glover, G. H. (1991). Phase-offset multiplanar (POMP) volume imaging: a new technique. Journal of Magnetic Resonance Imaging : JMRI, 1(4), 457–461. http://doi.org/10.1002/jmri.1880010410
5. Selective Excitation for Ultrashort Echo Time Imaging. (2012). http://doi.org/10.1002/9780470034590.emrstm1271
6. Pruessmann, K. P., Weiger, M., Börnert, P., and Boesiger, P. Advances in sensitivity encoding with arbitrary k-space trajectories. Magnetic Resonance in Medicine 4466 pp. 638–651 (2001).
7. Sutton, B. P., Noll, D. C., and Fessler, J. A. Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Transaction in Medical Imaging 2222 (2003), pp. 178–188.
Figures