4135
SWI+: A robust artifact-free SWI procedure with improved contrast1United Imaging of Health America, Inc., Houston, TX, United States, 2Shanghai United Imaging of Healthcare, Shanghai, China
Synopsis
A robust image reconstruction and processing strategy to achieve minimal susceptibility artifact for SWI is developed and demonstrated. With a multi-echo GRE data acquisition and a newly developed robust rapid phase unwrapping algorithm, an enhanced version of SWI imaging procedure, namely SWI+, is developed to address the pitfalls in classic SWI, which collects only one echo and simply employs high pass filtering on phase images.
Introduction
Classic susceptibility weighted imaging (SWI)1 combines both T2*W magnitude and susceptibility weighted phase masking to generate an image contrast sensitive to venous blood and iron/calcium deposition. However, the simple high-pass (HP) filtering process on the phase images inevitably leaves strong susceptibility artifacts originating from residual phase aliasing, frequently seen at air-tissue boundaries and/or veins/bleedings/iron-ridden tissues with strong susceptibility variations. Also the single echo data can only provide limited and sometimes misleading information. Therefore we aim to develop an advanced SWI procedure, namely SWI+, to address all such shortcomings of classic SWI.Method
SWI+ adopts a multi-echo GRE sequence for data acquisition. In general, there is little restriction on the scanning protocol, except that at least three echoes should be collected with equal ΔTE for best outcomes. With local IRB permit, healthy volunteer data were collected on a 3T system (uMR 770, UIH, Shanghai) using a 4-echo GRE sequence with following parameters: TE=3.8/9.4/15/20.6ms, ΔTE=5.6ms, TR=30ms, FA=15°, voxel size=0.58x0.58x1mm3, BW=280Hz/px, TA=5’38”. The SWI+ procedure was implemented using manufacturer’s ADEPT platform for inline reconstruction, which is demonstrated in Fig.1.
Firstly all channels’ data are combined for every echo, using adaptive coil combine method2 to obtain original phase images free of CUSP artifacts3.
Then a Δφ(r) image (also R2* map) with minimal noise variation corresponding to ΔTE is extracted by solving:
$$$min_{x}\sum_{n=1}^{N_{echo}-1}||M_{n+1}(r)-M_{n}(r)x||_2^2$$$, where $$$x=exp(-R_2^*\triangle TE+i\triangle\psi(r))$$$
Δφ(r) is then unwrapped to remove phase aliasing. In principle one can adopt any phase unwrapping algorithm, but we found that a robust region-growing voxel-wise unwrapping algorithm can retain the exact field information (both local and global) and is thus best for the next step. As a side note, ΔB(r) here is of high quality and SNR, and can be used as the input for any QSM calculation after background field removal and division by γB0ΔTE.
With ΔB(r) at hand, a fast unwrapping for each echo’s phase images can be done using the method proposed previously4. Compared to the simple field map estimated from merely the first two echoes as previously proposed4, ΔB(r) map obtained in this work is more accurate and of higher quality, thus can better serve the purpose of predicting the reference phase of each echo for more reliable unwrapping results.
The unwrapped phase images of each echo are then removed of the background phase and normalized to create SWI phase masks, and finally multiplied to corresponding magnitude images to generate echo-wise SWI images. We found that either LBV or vSHARP can result in similarly high quality local phase images and SWI images.
Results & discussion
Representative images at different stages of the SWI+ procedure are shown below.
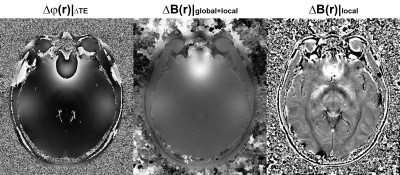
In Fig.2, the Δφ(r) image associated with ΔTE, and ΔB(r) before and after background field removal are displayed. As Δφ(r) was fitted from all echoes, it well represents the actual field distribution, albeit in a phase form corresponding to ΔTE. With competent phase unwrapping, a high quality field map can be obtained. On the other hand, the phase spatial continuity assumption is better satisfied in Δφ(r) due to its high SNR, allowing the adopted voxel-wise region growing phase unwrapping method to achieve very robust performance without any error propagation and yield an exact field distribution map of ΔB(r) (both global and local field). This pristine field map is crucial for ensuring the accuracy of the subsequent step of fast phase unwrapping for individual echo phase.
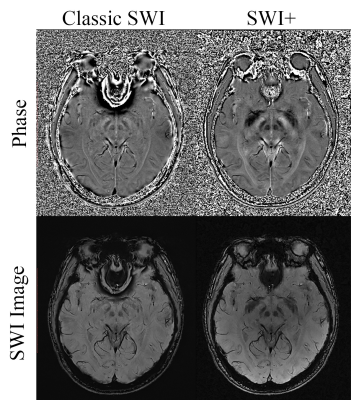
Fig.3 shows the comparison of the 4th echo results between SWI+ and classic SWI. Most noticeably, there’s virtually no residual phase aliasing in tissues near the air-tissue boundaries such as the nasal cavity in SWI+ phase images, while such aliasing are significant in HP filtered phase for classic SWI. This is the direct result of the high quality field map and the two-stage phase unwrapping strategy as described above. Secondly, SWI+ produces more salient image contrast in basal ganglia regions, as a dedicated background field removal algorithm can retain local field information with wider range of useful spatial frequency, while HP filtering will lead to a loss of such useful components. For brain regions with mild background field variation, both SWI+ and classic SWI yields virtually identical results, suggesting SWI+ mainly imposes improvement over classic SWI without any compromise.
Conclusion
We have demonstrated a renovated and practical SWI+ procedure to achieve artifact-free SWI results with focally improved image contrast. Moreover, as direct output of the process, the pristine local field and R2* maps can be directly used for subsequent susceptibility quantification analysis, which will be our future focus.Acknowledgements
No acknowledgement found.References
1. Haacke, E.M., et al., Susceptibility weighted imaging (SWI). Magn Reson Med, 2004. 52(3): p. 612-8.
2. Walsh, D.O., A.F. Gmitro, and M.W. Marcellin, Adaptive reconstruction of phased array MR imagery. Magn Reson Med, 2000. 43(5): p. 682-90.
3. Haacke, E.M., et al., Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging, 2015. 33(1): p. 1-25.
4. Ye. Y.Q., et al. Robust and fast phase unwrapping strategy to improve SWI quality. in Proceedings 25th Scientific Meeting, International Society for Magnetic Resonance in Medicine. 2017. Hawaii, USA.
Figures