4134
Respiration resolved imaging using continuous steady state multiband excitation with linear frequency sweeps1Centre for the Developing Brain, School of Imaging Sciences and Biomedical Engineering, Kings College London, London, United Kingdom, 2Centre for Medical Image Computing, University College London, London, United Kingdom
Synopsis
Free-breathing abdominal MRI is challenging due to the unpredictable and complex 3D deformation caused by respiration. We present a novel acquisition where short TR single-band or multiband slice acquisitions are swept smoothly across the anatomy of interest while preserving steady state conditions. This is achieved by adding a frequency offset to successive RF pulses shifting the excited slice by fraction of slice thickness for each acquired k-space. This creates a highly efficient acquisition with high redundancy in respiration. A method is presented to produce 4D respiration-resolved volumes from this data and examples of human placenta and kidney are presented.
Introduction
Three dimensional (3D) imaging of abdominal organs is a challenging application of MRI requiring encoding of large volumes in the presence of complex, non-rigid motion induced by respiration, which is only quasi-periodic. Previous methods seek to freeze motion by breath holding, which limits imaging time, or to accommodate movement through gating techniques and/or high acceleration factors.1, 2
Slice selective short-TR sequences, such as balanced steady state free precession (bSSFP) can be fast enough to freeze abdominal motion, but each new slice location requires a start-up phase to establish the required steady state. The result is motion free slices that are geometrically inconsistent because of changes in anatomical position during time needed to cover a complete 3D region of interest. In this work we explore a “sweep” concept to resolve this problem and test it using bSSFP methods.
Methods
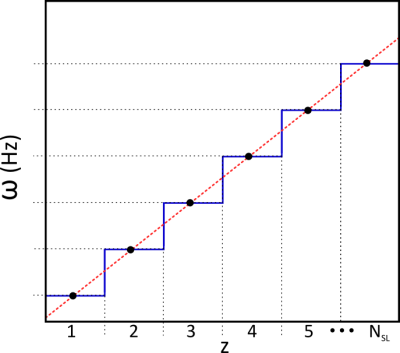
A bSSFP sequence was modified to introduce a small frequency shift, Δω=(δz/Δz).(BW/NPE), for each RF pulse, where δz is a slice shift and Δz is the slice thickness, both in mm, BW is the pulse bandwidth and NPE is the number of phase encode steps. Thus each new RF pulse excites a slightly shifted slice, but fractional volume of newly excited tissue is tiny. The leading edge of the slice is continuously in transition towards a steady state. In the proposed application, the slice moves slowly to ensure that it takes a time that is at least as long as a typical respiratory period to translate by one slice thickness.
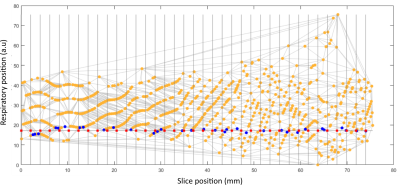
Following conventional image reconstruction, images were assigned a respiratory position based on the separation of appropriately selected edges of the abdomen (AP for a sagittal slice or LR for coronal). Each slice was then projected into a respiratory-slice position space and, for any respiratory position, a consistent stack was defined by selecting the closest vertex of a Voronoi diagram connecting all points (Fig 2.). The extremes of respiratory position were removed by excluding positions >±1.2std.dev from the mean.
The method was tested on a 3T Philips Achieva system with a 32-channel cardiac coil with 1.5x1.5mm in-plane resolution; 360x360mm FOV; 3mm slice thickness; pulse BW=815Hz. The effect on signal strength and stability of varying δz was explored with a phantom (T1=2200ms/T2=180ms) scanned using bSSFP; TR/TE 7.5/3.8, 50° flip, SENSE 1;halfscan 0.7. Free-breathing in-vivo tests were performed on two healthy adult volunteers and two pregnant subjects (28 and 34 weeks); all subjects gave informed consent. For the examples presented parameters were: For placenta: 256x3mm slices; 0.5mm shift/slice; coverage 131mm; single-band RF pulses; flip 90°; halfscan 0.7; SENSE 2; TE/TR=3.9/7.9ms; time/slice=643ms. For kidney: 768x3mm slices; 0.2mm shift/slice; coverage 154mm; multiband factor 3 using a z-blip approach3; flip 70°; halfscan 0.7; TE/TR = 4.9/9.9ms; time/slice = 503ms. The slices were sorted into 8 respiratory volumes of slice thickness 3mm (placenta) and 2mm (kidney).
Results
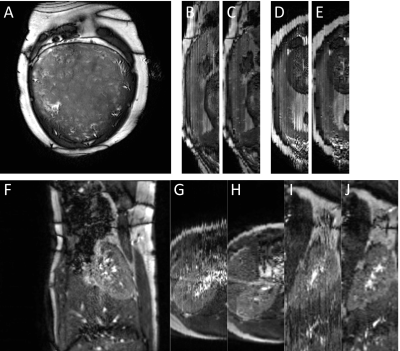
Phantom studies produce bSSFP images free of motion artefacts up to the largest shift tested of (δz/Δz)=1. There was a linear increase of signal with slice shift from 1.2-8.2mm/pulse. With the signal normalised to δz=0 the slope for this relationship is approximately δz/Δz. In-vivo sweep rates ((δz/Δz)>0.3) produced pronounced respiration related signal fluctuations. For in-vivo sorted volumes (Fig. 3) individual slices are uncorrupted by respiratory motion (Fig.3A,F). However, in the through plane direction respiration induced movement disrupts the ability to resolve organs. Placenta imaging was performed coronal to the mother with orthoganol planes showing corruption (Fig.3B,D). After sorting the volumes appear more consistent (Fig.3C,E). There are however residual artefacts in these examples due to insufficient sampling. The multiband acquisition in the kidney (Fig. F-J) shows the benefit of denser sampling in this domain.Discussion
The existing method closest to the presented Sweep approach is continuous moving table acquisition4,5, although Sweep is not limited to imaging in the axial plane and the motivation is quite different. A consequence of the RF sweep method is blurring the slice profile in the kz dimension. However, this effect is minimised by the deliberate choice of tiny fractional slice shifts to ensure that a diversity of respiratory states occur during the time taken to move one complete slice thickness. This requirement could limit spatial coverage, but use of multiband acquisition is highly effective in avoiding this.Conclusion
We present an efficient rapid imaging technique for dense sampling in respiration and slice location that enables free-breathing respiration-resolved imaging of deformable organs. The resulting consistent volumes could be of utility in their own right, and may also provide a basis for a potential next generation slice-to-volume registration6 approaches employing deformable models.Acknowledgements
This work received funding from the European Research Council under the European Union’s Seventh Framework Programme [FP7/20072013]/ERC grant agreement no. 319456 (dHCP project), the NIH Human Placenta Project grant [1U01HD087202-01], and was supported by the Wellcome EPSRC Centre for Medical Engineering at Kings College London [WT 203148/Z/16/Z], MRC strategic grant [MR/K006355/1] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of HealthReferences
(1) Yoon, Jeong Hee, et al. "Clinical Feasibility of Free-Breathing Dynamic T1-Weighted Imaging With Gadoxetic Acid–Enhanced Liver Magnetic Resonance Imaging Using a Combination of Variable Density Sampling and Compressed Sensing." Investigative Radiology 52.10 (2017): 596-604.
(2) Murphy, Ian Gavin, et al. "Comparison of breath-hold, respiratory navigated and free-breathing MR elastography of the liver." Magnetic resonance imaging 37 (2017): 46-50.
(3) Price, Anthony , et al. “Accelerated Cine Imaging of the Heart using Blipped Multiband SSFP” Proc. Intl. Soc. Mag. Reson. Med. 25 (2017)
(4) Shankaranarayanan, Ajit, et al. "Helical MR: continuously moving table axial imaging with radial acquisitions." Magnetic resonance in medicine 50.5 (2003): 1053-1060.
(5) Börnert, Peter, and Bernd Aldefeld. "Principles of whole‐body continuously‐moving‐table MRI." Journal of Magnetic Resonance Imaging 28.1 (2008): 1-12.
(6) Kuklisova-Murgasova, Maria, et al. "Reconstruction of fetal brain MRI with intensity matching and complete outlier removal." Medical image analysis 16.8 (2012): 1550-1564.
Figures