4133
Enhancing Spatial-Temporal Resolution in Simultaneous Multi-Slab Echo Volumar Imaging1Neurology, Physics and Astronomy, University of New Mexico, Albuquerque, NM, United States, 2Center for Magnetic Resonance Research, Radiology, University of Minnesota, Minneapolis, MN, United States, 3Center for Advanced Imaging, Innovation and Research (CAI2R), New York University School of Medicine, New York, NY, United States, 4Neurology, University of New Mexico, Albuquerque, NM, United States
Synopsis
We develop multi-band echo-volumar-imaging (MB-EVI) by combining multi-slab encoding with EVI to investigate BOLD sensitivity, resting-state connectivity in different frequency bands, and spatial-temporal resolution limits. We also study the effect of compressed sensing (CS) reconstruction on fMRI sensitivity. Mapping of major resting-state networks with 3mm3 voxel size was feasible in multiple frequency bands. High spatial resolution (2mm3) improved delineation of neuroanatomy and enabled sensitive mapping task-based activation. CS with 2.4-fold undersampling showed negligible loss in image quality and moderate region-specific losses in BOLD sensitivity. MB-EVI provides flexibility for maximizing spatial-temporal resolution, volume-coverage and BOLD-sensitivity for mapping task-activation and functional connectivity.
Introduction
Recent advances in fMRI acquisition rates, using multi-band (simultaneous multi-slice) encoded EPI (MB-EPI)1, magnetic resonance encephalography (MREG), and multi-slab echo-volumar imaging (MEVI)2, have played an important role in improving sensitivity for mapping functional connectomics3,4,5 and for detecting task-based and resting state fMRI signal changes at frequencies above 0.2Hz. Further increases in spatial-temporal resolution are desirable for unaliased sampling of physiological signal pulsation at high spatial resolution and for reducing sensitivity to rapid movement, which requires combination of multiple complementary acceleration approaches. In this study, we develop multi-band EVI by combining MEVI2 with simultaneous multi-slab encoding, which in a previous feasibility study has shown considerable acceleration for task-based fMRI at moderate spatial resolution6. We additionally investigate the feasibility of compressed sensing reconstruction of undersampled pseudo k-space data after multi-band and MEVI reconstruction in preparation for a full integration of these approaches. We investigate spatial-temporal resolution limits of this approach, BOLD sensitivity, and mapping of resting-state connectivity in different frequency bands.Methods
MB-EVI was developed based on multi-echo EPI7,8 and simultaneously excites 2 or 3 slabs with EVI encoding within slabs, using in-plane 4-fold GRAPPA undersampling and 6/8 partial-Fourier (PF) acquisition along ky or ky-kz. Reconstruction was performed using regularized ‘leak-block’ slab-GRAPPA multiband-reconstruction, which limits the slab cross-talk between excitation shots. Compressed sensing was implemented by variable-density random undersampling based on a Poison disk distribution on ky-kz of the full slab k-space data and reconstructed with SPARSE-SENSE9 and temporal PCA6 as sparsifying transform. CS reconstruction parameters were chosen to minimize spatial-temporal smoothing. The through-plane reconstruction combined the inner slices from each of two adjacent slabs that overlapped the gaps. Task-based (motor/visual) and resting-state data were acquired in 10 healthy controls on a 3T-scanner using 32-channel head coil. Informed consent was obtained. MB-EVI was acquired with a range of isotropic spatial resolutions (2, 2.5, 3 and 6 mm), number of slabs (4-9) and flip angles (10o to Ernst angle for gray matter). For comparison, additional data were acquired using MB8-EPI and MEVI (without MB acceleration). Task- and resting-state fMRI analysis was performed using the TurboFIRE fMRI software tool5,10 with motion correction, matched isotropic Gaussian spatial filter and spatial normalization into MNI space. Seed-based sliding window correlation analysis (SCA) was performed with 8s moving average temporal low pass filter, 15s sliding window correlation analysis with running mean and regression of 8 signal time courses from 6 rigid body motion parameters and WM and CSF ROIs. RSNs were mapped using unilateral Brodmann area (BA) based seed regions (auditory- AUN–BA41,42; default-mode-DMN–BA7,31; sensorimotor-SMN–BA01-03, visual-VSN–BA17, language-LAN-BA44,45 (Broca) and BA22,39,40 (Wernicke).Results
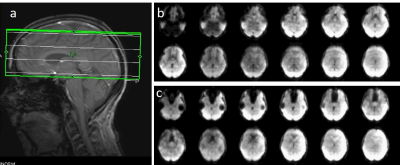
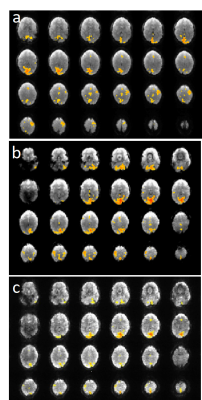
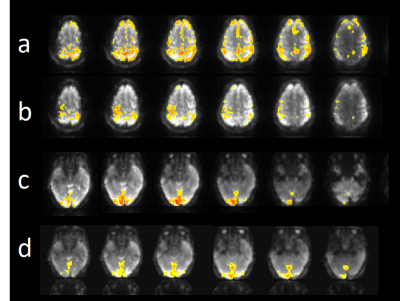
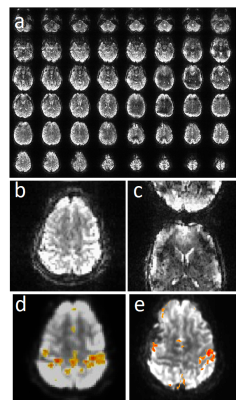
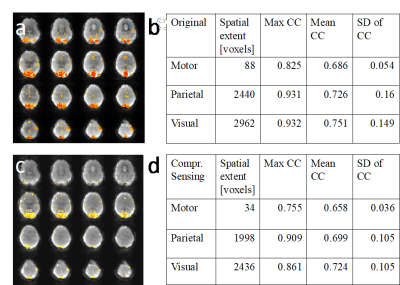
Susceptibility related signal losses in orbital frontal and temporal regions with MB-EVI was comparable to MEVI. Depending on the orientation of the kz-encoding gradients, which counteracted local gradients (“Z-shimming”), signal dephasing in orbital-frontal cortex or in temporal lobe was substantially reduced compared with MB-EPI at comparable effective echo time (TE) (Figure 1). Respiration related signal fluctuation increased with kz-encoding, limiting BOLD-sensitivity relative to MB8-EPI (Figure 2). Sensitive mapping of major resting-state networks (RSNs) with 3mm3 voxel size was feasible in multiple frequency bands (Figure 3). High spatial resolution (2mm3 voxel) MB-EVI improved delineation of neuroanatomy and enabled sensitive mapping task-based activation (Figure 4). CS with 2.4-fold retrospective undersampling relative to the fully sampled k-space showed negligible loss in image quality and moderate region-specific losses in BOLD sensitivity (Figure 5). The 3.1-fold undersampling resulted in similar image quality, but increased spatial blurring of activation patterns.Discussion
MB-EVI provides flexibility for maximizing spatial-temporal resolution, volume-coverage and BOLD-sensitivity for mapping task-activation and functional connectivity. Gains in spatial-temporal resolution were limited by increasing signal instability with MB acceleration, sensitivity to respiratory signal pulsation, and increasing TE with decreasing voxel size. Some of these limitations could be potentially circumvented by a combination with CS, which will enable higher in-plane accelerations. Further limitations of this approach include sensitivity loss at slab interfaces due to slab profile imperfections and T1 saturation, and Gibb’s ringing in the slice direction. Future work will include: integration of CAIPI-encoding to reduce inter-slab crosstalk, kz-segmented acquisition to reduce TE, integration of CS with randomized undersampling reconstruction, and nonlinear inversion for slab profile encoding (NPEN)11.Conclusions
Combining MEVI with simultaneous multi-slab encoding rectifies primary inefficiencies of current 2D encoding when many slices are needed and the temporal constraints of the slab-segmented EVI approach. Further work is needed to improve image quality at slab boundaries and temporal stability.Acknowledgements
Research reported in this publication was supported by NIH grant number R21EB018494. We thank Diana South, and Catherine Smith for assistance with data acquisition.References
[1] K. Setsompop, B. A. Gagoski, J. R. Polimeni, T. Witzel, V. J. Wedeen, and L. L. Wald, "Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty," Magn Reson Med, vol. 67, pp. 1210-1224, May 2012, 3323676.
[2] S. Posse, E. Ackley, R. Mutihac, J. Rick, M. Shane, C. Murray-Krezan, M. Zaitsev, and O. Speck, "Enhancement of temporal resolution and BOLD sensitivity in real-time fMRI using multi-slab echo-volumar imaging," Neuroimage, vol. 61, pp. 115-130, May 15 2012, 3342442.
[3] D. A. Feinberg, S. Moeller, S. M. Smith, E. Auerbach, S. Ramanna, M. Gunther, M. F. Glasser, K. L. Miller, K. Ugurbil, and E. Yacoub, "Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging," PLoS One, vol. 5, p. e15710, 2010, 3004955.
[4] S. M. Smith, K. L. Miller, S. Moeller, J. Xu, E. J. Auerbach, M. W. Woolrich, C. F. Beckmann, M. Jenkinson, J. Andersson, M. F. Glasser, D. C. Van Essen, D. A. Feinberg, E. S. Yacoub, and K. Ugurbil, "Temporally-independent functional modes of spontaneous brain activity," Proc Natl Acad Sci U S A, vol. 109, pp. 3131-3136, Feb 21 2012, 3286957.
[5] S. Posse, E. Ackley, R. Mutihac, T. Zhang, R. Hummatov, M. Akhtari, M. Chohan, B. Fisch, and H. Yonas, "High-speed real-time resting-state FMRI using multi-slab echo-volumar imaging," Front Hum Neurosci, vol. 7, p. 479, 2013, 3752525.
[6] L. Chen, Feinberg, D., "Simultaneous Multi-Slab Echo Volume Imaging: comparison in sub-second fMRI," in Proc: International Society of Magnetic Resonance in Medicine, Salt Lake City, Utah, USA, 2013, p. 303.
[7] O. Speck and J. Hennig, "Functional imaging by I-0- and T-2*-parameter mapping using multi-image EPI," Magnetic Resonance in Medicine, vol. 40, pp. 243-248, Aug 1998.
[8] O. Speck, T. Ernst, and L. Chang, "Biexponential modeling of multigradient-echo MRI data of the brain," Magn Reson Med, vol. 45, pp. 1116-1121, Jun 2001.
[9] R. Otazo, D. Kim, L. Axel, and D. K. Sodickson, "Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI," Magn Reson Med, vol. 64, pp. 767-776, Sep 2010, 2932824.
[10] S. Posse, F. Binkofski, F. Schneider, D. Gembris, W. Frings, U. Habel, J. B. Salloum, K. Mathiak, S. Wiese, V. Kiselev, T. Graf, B. Elghahwagi, M. L. Grosse-Ruyken, and T. Eickermann, "A new approach to measure single-event related brain activity using real-time fMRI: Feasibility of sensory, motor, and higher cognitive tasks," Human Brain Mapping, vol. 12, pp. 25-41, Jan 2001.
[11] W. Wu, P. J. Koopmans, R. Frost, and K. L. Miller, "Reducing slab boundary artifacts in three-dimensional multislab diffusion MRI using nonlinear inversion for slab profile encoding (NPEN)," Magn Reson Med, vol. 76, pp. 1183-1195, Oct 2016, PMC4854328.
Figures