4127
fMRI at 7 Tesla with 0.5mm Isotropic Resolution and Full Field of View1Center of medical physics and engineering, University of Erlangen-Nuremberg, Erlangen, Germany, 2Siemens Healthcare GmbH, Erlangen, Germany, 3University of Glasgow, Glasgow, United Kingdom, 4Siemens Healthcare United Kingdom, Glasgow, United Kingdom, 5Siemens Shenzen Magnetic Resonance Ltd., Shenzen, China
Synopsis
fMRI protocols at ultra-high field typically use pixel sizes below 1mm. With single-shot EPI, this results in a prolonged readout train relative to the T2* decay time, resulting in image blurring and a limit to the true resolution that can be achieved. This effect can be mitigated by using multi-shot EPI to reduce the echo-train length, but this is associated with a reduction in temporal SNR. Previous work demonstrated a less severe reduction in temporal stability with readout-segmented EPI than with interleaved EPI. This study investigates the application of readout-segmented EPI to ultra-high resolution fMRI of the motor cortex.
Introduction
Readout-segmented (rs) echo-planar Imaging (EPI)1 is widely used in high-resolution diffusion MRI2 due to its capability to shorten the echo train, thereby reducing distortions and blurring. Previous work3 has shown similar benefits when rs-EPI is used for functional Magnetic Resonance Imaging (fMRI) studies at ultra-high field (UHF). The higher SNR available at UHF offers the possibility of increasing the spatial resolution, but this is confounded by the associated increase in readout time and the shortening T2* values as the magnetic field increases. In the case of single-shot EPI, this places a limit on the true spatial resolution that can be reached. This study explores the capability of rs-EPI to overcome these limitations and to perform UHF fMRI at a spatial resolution that is beyond that currently achievable with single-shot methods.Methods
All data were acquired with a prototype sequence on a MAGNETOM Terra 7T system (Siemens Healthcare GmbH, Erlangen) with a single-channel-transmit, 32-channel-receive head coil (Nova Medical, Wilmington MA, USA). The local ethics committee granted approval for the study.
A modified rs-EPI sequence was used, which supported in-plane parallel imaging using GRAPPA4 and simultaneous multislice (SMS) imaging using blipped-CAIPIRINHA5. The sequence was designed to allow data sampling during the application of gradient blips in the phase-encoding (PE) and slice-encoding directions. This improved the sampling efficiency of the sequence by minimizing the dead time during the EPI readout. As described in previous work6, the corresponding deviations from a standard EPI k-space trajectory only have a limited effect on image quality and slice-separation algorithms and no correction was made for these effects in this study.
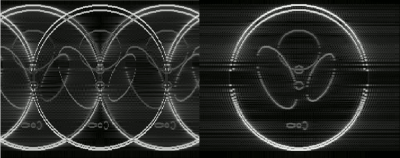
In addition to an improved temporal stability compared to interleaved EPI, the readout-segmented approach has potential advantages with respect to its motion sensitivity. This potential benefit was investigated using a Shepp-Logan phantom to simulate the effect of a positional shift of 3 pixels when acquiring the central shot of each multi-shot data set.
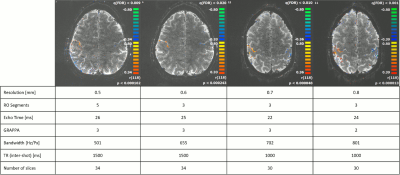
As shown in the table in fig 2, data were acquired at four spatial resolutions, corresponding to pixel sizes between 0.5mm and 0.8mm for a FOV of 192mm. Depending on the resolution, the number of readout segments (or shots) was varied between 3 and 5 and the in-plane acceleration factor was varied between 2 and 3. In all cases, a slice-acceleration factor of 2 was used. The short echo spacing of the readout-segmented readout allowed TE values in the range 22ms to 26ms, despite the high spatial resolution. fMRI was performed using a standard finger-tapping paradigm to activate the motor cortex. Data analysis was performed using Brain-Voyager (Brain Innovation, Maastricht, Netherlands) with a minimal post-processing protocol consisting of motion correction, high-pass filtering and slice-time correction.
Results
Fig. 1 shows the simulated effect of motion on interleaved and rs-EPI. In each case, the images show the difference between the corrupted and uncorrupted data set. In the case of interleaved EPI, motion results in more severe folding artefacts whereas with readout-segmentation, motion produces a blurring of the image. Fig. 2 compares the results of the finger-tapping experiments for the different spatial resolutions, together with the acquisition parameters used in each case. As shown in the corresponding zoomed activation maps of fig. 3, all acquisitions resulted in a detailed pattern of activation along the motor cortex.Discussion
The reduced severity of motion artefacts and the improved temporal stability of rs-EPI compared to interleaved EPI makes it an attractive option for fMRI studies at UHF when operating at spatial resolutions that are beyond the limits of single-shot EPI. The ultra-high resolution fMRI data acquired in this study have demonstrated the ability of the technique to depict clear BOLD signal changes in the motor cortex at sub-millimeter isotropic pixel dimensions at 7T. Further work is required to determine whether there is sufficient sensitivity to detect the smaller signal changes associated with cognitive tasks. The multi-shot approach does have the disadvantage of requiring multiple TR periods to acquire each volume, which might be problematic for some fMRI paradigms. In some cases, it might however be possible to reduce volume acquisition times by using a higher slice-acceleration factor with a reduced in-plane acceleration factor compared to the single-shot case.Conclusion
This study has demonstrated the feasibility of performing high-resolution fMRI with an isotropic resolution of 0.5mm at 7T using multi-shot EPI based on readout segmentation. The technique promises to enable fMRI studies with higher spatial resolutions than currently in use and might have a significant impact on neuroscience studies that focus on layer-specific cortical activity7.Acknowledgements
No acknowledgement found.References
1. Mansfield P. Multi-planar image formation using NMR spin echoes. J. Phys. C: Solid State Phys. 1977;10(3):L55
2. Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med. 2009 Aug;62(2):468-75. doi: 10.1002/mrm.22024.
3. Robison R, Newton A. Evaluation of Readout-Segmented EPI for Use in fMRI at 7T. Proceedings of ISMRM. 2013.
4. Griswold M, Jakob P, Heidemann R, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002;47(6):1202-1210.
5. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-24.
6. Liebig P, Heidemann R, Hensel B, Porter D. Variable-blipped-EPI (VB-EPI) for Lower Acoustic Noise and Higher Efficiency with non-Cartesian iterative Reconstruction. Proceedings of ISMSM. 2017
7. Muckli L, De Martino F,Vizioli L, Petro L, Smith F, Ugurbil K, GoebelR, Yacoub E. Contextual feedback to superficial layers of V1. Current Biology. 25(20):2690-5.
Figures