4126
Clinical Evaluation of Pile-up and Ripple Artifact Suppression Near Metal by Alternating Gradients1Electrical Engineering, Stanford University, Stanford, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States
Synopsis
Multi-Spectral Imaging techniques have been shown to significantly reduce metal-induced artifacts. However, they often suffer from pile-up/ripple artifacts near metal, where metal-induced-off-resonance gradients “cancel” the frequency-encoding gradient. We have previously proposed a method to reduce these artifacts by combining two acquisitions with alternating-sign readout and slice-select gradients. Here we demonstrate that the alternating-gradient method is compatible with robust PCA, which provides 2-fold acceleration beyond parallel imaging and half-Fourier acquisition to compensate for the additional acquisition. Our study shows that the alternating-gradient method can significantly reduce pile-up/ripple artifacts in volunteers with hip and knee arthroplasties, and provides additional diagnostic advantages compared to the standard sequence.
Purpose
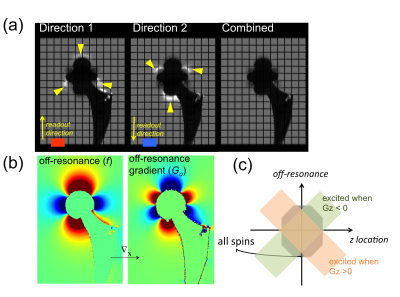
Multi-Spectral Imaging (MSI) techniques, such as SEMAC1 and MAVRIC-SL2, significantly reduce metal-induced artifacts, but often suffer from pile-up and ripple artifacts in the vicinity of metal where metal-induced off-resonance gradients cancel the readout gradient3,4. The regions affected by these artifacts depend on the direction of readout gradients, as demonstrated in the simulations (Fig. 1a,b). In spatial-selective MSI, spins with high off-resonance frequencies at edge slice (z) locations may be unexcited with one slice-select gradient direction, causing signal voids in the images (Fig. 1c). With the slice-select gradient inverted, these signal voids appear in different locations. Therefore, we have previously proposed a method to reduce pile-up/ripple artifacts and signal voids by combining two MSI acquisitions with alternating-sign readout and slice-select gradients5. Here we integrate acceleration using robust PCA6 with this approach, and study its artifact correction and diagnostic potential in patients.Methods
The alternating-gradient method was evaluated in 7 volunteers (6 with total hip replacements and 1 with a total knee replacement) using coronal MAVRIC-SL proton density-weighted imaging. All scans were performed on GE 3T MRI systems with 24 spectral bins spanning ±12kHz and readout bandwidth of ±125kHz. Other parameters of the hip scans include: matrix size=384x256 or 512x384, FOV=40cm x 40cm, number of slices=24-44, slice thickness=4.0mm. Other parameters of the knee scan include: matrix size=256x256, FOV=36cm x 36cm, number of slices=40, slice thickness=4.0mm.
In 3 hip cases and 1 knee case, standard acceleration (2x2 uniform under-sampling for parallel imaging, half-Fourier acquisition, R=8-9) was applied, and scan times were 6.0-9.6min for each direction. In 3 hip cases, the acquisitions of both directions were accelerated by 2x in addition to standard acceleration (R=17, scan times=4.5-4.9min for each direction), and reconstructed by robust PCA6 before combination of the two directions.
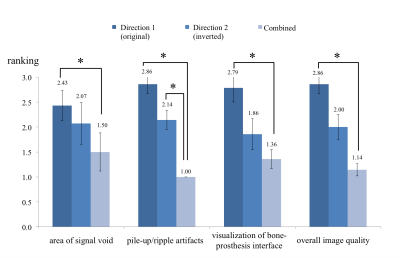
In each scan, the standard MAVRIC-SL sequence was run first (Gx direction S+/I-, Gz direction A+/P-, labeled as Direction-1), and then repeated with readout, slice-select and VAT gradients all inverted (Gx direction S-/I+, Gz direction A-/P+, labeled as Direction-2). Translational registration was performed before the combination. The three sets of images in each case were randomly ordered and ranked by an experienced MSK radiologist (1: best; 2: neutral; 3: worst) in four categories: 1) area of signal void, 2) pile-up/ripple artifacts, 3) visualization of bone-prosthesis interface, 4) overall image quality. The images were also assessed for visualization of abnormalities near implants.
Results & Discussion
As shown in Fig.2, the combined images were ranked highest in all categories on average. An interesting observation was that the gradient-inverted sequence (Direction-2) was better than the original sequence (Direction-1) on average, because total hip replacements tend to induce larger off-resonance gradients above the femoral head that cancel the readout gradient of Direction 1 and cause more severe pile-up artifacts. Two-sided Wilcoxon signed rank tests showed significant differences in all categories between Direction-1 and Combined, and in the “pile-up/ripple artifacts” category between Direction-2 and Combined (p<0.05).
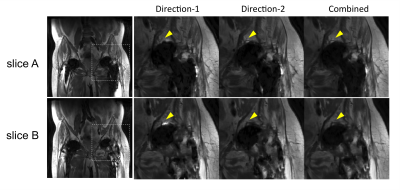
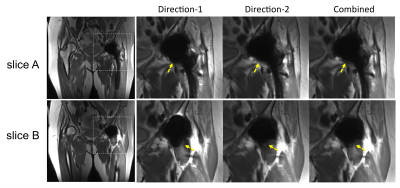
Periprosthetic signal abnormality was identified in the superior acetabulum in two cases (Fig.3 and Fig.4). The abnormality was visualized in Combined in both cases, but was not clearly visualized due to pile-up artifacts in Direction-1 in one case (Fig.3) or due to signal voids in Direction-2 in the other case (Fig.4). This observation suggests the necessity of acquiring both gradient directions to avoid missing abnormalities near the tissue-prosthesis interface. Although one gradient direction may have smaller artifacts overall than the other, the best direction often changes in different slices or regions.
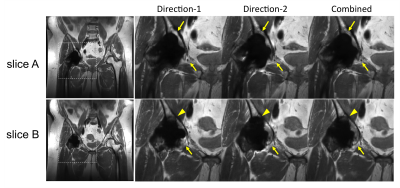
In only one case out of 7 studies, the combined image was ranked worse in “overall image quality” category than one individual direction (Fig.5). In this case, the combined image had slightly larger areas of signal loss near the tissue-prosthesis interface compared with Direction-2 (dashed arrows) in one slice; as a weighted combination of two directions, the combined image in this example could be affected by the worse direction when combination weights had small errors due to inter-scan motion or inaccurate off-resonance mapping. The combination method could be further improved to reduce scan time and improve off-resonance field mapping for a more robust performance.
Conclusion
Our preliminary results showed that the alternating-gradient acquisitions reduced pile-up/ripple artifacts, signal voids and improved visualization of bone-prosthesis interface significantly compared with the standard acquisition.
The gradient-inverted acquisition (Gx direction S-/I+, Gz direction A-/P+) performed better than the original gradient direction (Gx direction S+/I-, Gz direction A+/P-) overall, and could be considered as an alternative option of acquiring both gradient directions if scan time is limited.
Acknowledgements
NIH: R01 EB017739, R21 EB019723, P41 EB015891; research support from GE Healthcare.References
[1] Lu et. al, MRM 62:66-76, 2009; [2] Koch et. al, MRM 65:71-82, 2011; [3] Koch et. al, MRM 71: 2024-34, 2014; [4] den Harder et. al, MRM 73: 318-24, 2015; [5] Shi et. al, ISMRM 2017, p. 574; [6] Levine et al., MRM DOI: 10.1002/mrm.26819, 2017.Figures