4112
Implementation of a control system for prospective respiratory motion correction during Cardiovascular MR imaging1Division of Biomedical Engineering, Department of Human Biology, University of Cape Town, Cape Town, South Africa, 2Cape Universities Body Imaging Centre (CUBIC), University of Cape Town, Cape Town, South Africa, 3Department of Medicine, University of Cape Town, Cape Town, South Africa
Synopsis
Navigated free-breathing techniques are commonly used during cardiovascular MR (CMR) acquisitions when breath-holding techniques are not a viable option. Navigated free-breathing techniques with acceptance windows suffer from much longer scan times. A control system using an adaptive Kalman filter was developed to continuously update the slice position throughout the imaging segment during free-breathing CMR, allowing the acquisition to continue throughout the respiratory cycle, regardless of diaphragm position, thereby improving respiratory efficiency and reducing scan times.
Introduction
Respiratory motion of the heart poses a challenge for high resolution CMR imaging. Breath-holding to compensate for respiratory motion, limits scan times, and as such signal-to-noise ratio and resolution.1 Several free breathing techniques have been proposed to monitor and correct for respiratory motion in real time using navigators.2 Diaphragmatic navigator gating with an acceptance window is commonly used but the technique has a low respiratory efficiency which is further compromised by respiratory drift. Prospective slice following has been implemented to enable larger gating windows and increased respiratory efficiency. This technique uses the navigator position immediately prior to the imaging segment to determine the slice position to be used during the segment. The slice following becomes less accurate as the duration of the imaging segment increases due to the navigator data becoming more temporally outdated.2 In this work, we have implemented a prospective motion correction control system technique based on an adaptive Kalman filter, in a TRUFI sequence, to continuously update the slice position throughout the imaging segment.3Methods
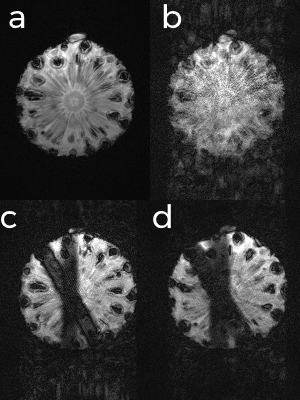
The TRUFI sequence was modified to allow multiple 90°/180° navigators to be run prior to the imaging segment (Figure 1). The data from the navigators are used by the control system as the input of a predictor estimator, the output of which predicts the position of the diaphragm throughout the imaging segment when no navigator data are available.3 Phantom and in vivo scans were performed on a 3T Siemens Skyra (Siemens AG, Erlangen). A pineapple and water phantom were imaged with a 32-channel head coil on a moving rig while undergoing simulated breathing (Figure 2). The rig could be moved in the superior-inferior direction to simulate tidal breathing. Acquisitions were performed using three sequences: an ECG-triggered TRUFI sequence, a navigated TRUFI sequence with a ±3mm acceptance window, and the modified navigated TRUFI sequence with the prospective motion correction control system. The scanning protocol was as follows for all the sequences: TR/TE=200/2.27ms, effective TR of 1000ms due to ECG gating; 70° flip angle; 256×256 matrix size; slice thickness=5mm; 24 segments per TR; 1 slice; GRAPPA 2. All slices were positioned axially. The navigator was placed over the interface between the pineapple and the water phantom which when moved would simulate the motion of a diaphragm. The peak-to-peak amplitude of the oscillation was 15mm and the frequency of the oscillation was approximately 0.2Hz, a normal adult respiratory rate.4 A healthy volunteer was then imaged with an 18-channel flex coil and a 32-channel spine array coil using the same sequences as previously described.Results
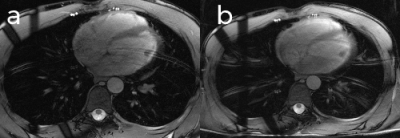
Figure 3 shows the results of the acquisitions on the pineapple-water phantom. In Figure 3b the image quality is badly degraded and most of the detail of the pineapple has been lost due to motion artefacts. In Figure 3c the image quality is greatly improved due to the use of the acceptance window which discards data acquired when the diaphragm is not within a specific range. Figure 3d shows the same amount of motion as in Figure 3c, however the acceptance window is set to have a 100% acceptance rate and the motion is prospectively corrected using the control system. Scanning times for Figures 3c and 3d were 22 and 8 effective TRs, respectively. Images acquired from a healthy volunteer using the ±3mm acceptance window (Figure 4a) and the control system to prospectively correct the breathing motion (Figure 4b) demonstrate similar image quality.Discussion
For the phantom scans, the prospective motion correction control system shows improvement over the uncorrected image, and similar image quality to the acceptance windowed acquisition, in a scan time that is 64% shorter than that of the latter. Many structures that were lost during the uncorrected acquisition are regained by use of the control system and ghosting artefacts present in both the uncorrected and acceptance windowed acquisitions are reduced. However, finer details that are visible in the stationary acquisition, especially the core of the pineapple, are poorly visualised in all other acquisitions. In vivo images acquired using the acceptance window and prospective motion correction control system both showed motion artefacts caused by breathing. Figure 5 shows the navigator trace produced by the prospective motion control system, showing that 100% respiratory efficiency was achieved.Conclusion
The prospective motion correction control system demonstrates significant improvement over the uncorrected acquisition, and similar image quality to the acquisition with a ±3mm acceptance window, a commonly used window size in CMR. Furthermore, the scan time of the prospective motion corrected acquisition was reduced by almost a factor of three when compared with the acceptance windowed acquisition.Acknowledgements
This study was funded by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South AfricaReferences
1. Holland, A. E., Goldfarb, J. W., & Edelman, R. R. (1998). Diaphragmatic and cardiac motion during suspended breathing: preliminary experience and implications for breath-hold MR imaging. Radiology, 209(2), 483–489.
2. Sachs, T. S., Meyer, C. H., Hu, B. S., et al. (1994). Real-time motion detection in spiral MRI using navigators. Magnetic Resonance in Medicine, 32(5), 639–45.
3. Burger, I. H., Keegan, J., Meintjes, E., et al. Prospective diaphragm position prediction for Cardiac MR using multiple navigators. Proc ISMRM-ESMRMB 18, ISSN# 1545-4428, Stockholm, Sweden, 1-7 May 2010. (#5013).
4. Lindh, W. Q., Pooler, M., Tamparo, C. D., et al. (2009). Vital Signs and Measurements. In Delmar’s Comprehensive Medical Assissting: Administrative and Clinical Competencies (4th ed., pp. 563–569).
Figures