4109
Comparison of three surrogate-based respiratory motion correction methods for 3D high resolution cardiac MRI1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Siemens Healthcare, Erlangen, Germany, 3King's College London, Division of Imaging Sciences and Biomedical Engineering, London, United Kingdom
Synopsis
Respiratory motion correction has been proposed to improve scan efficiency and ensure high image quality for 3D cardiac MRI. Nevertheless, these techniques often require dedicated data acquisition and cannot necessarily capture intra-cycle variations of the breathing. Here we compare three different surrogate signals (“pilot tone”, respiratory belt and MR-navigator) for a surrogate-based motion correction approach which provides motion information with high temporal resolution and can be combined with a wide range of different MR acquisition schemes. The temporal stability of these surrogates is assessed and motion correction of a 3D cardiac MR scan is demonstrated.
Introduction
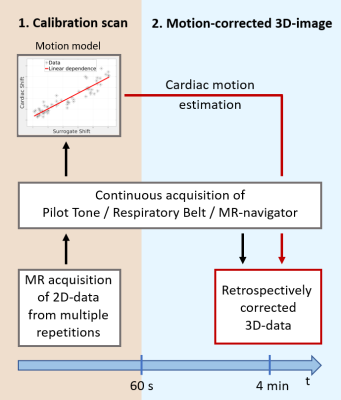
When acquiring 3D high-resolution cardiac MR-data, respiratory motion can lead to strong motion artefacts and impair the quality of reconstructed images. Commonly, respiratory gating techniques are used to minimize respiratory motion artefacts but they suffer from low scan efficiency.1-2 Respiratory motion correction approaches have been proposed to overcome this problem.3-6 Nevertheless, they often require dedicated data acquisition and cannot necessarily capture intra-cycle variations of the breathing. Here we present an approach that uses motion-surrogates to obtain motion correction information with high temporal resolution. A short pre-scan is used to calibrate the surrogate signals to the respiratory motion of the heart. For the subsequent 3D-data acquisition the surrogate is then used for motion-correction (Fig 1). A comparison of three different surrogate signals (“pilot tone”7, respiratory belt and MR-navigator) is carried out and the results of motion correction are presented.Methods
An overview of the calibration and motion correction method is given in Figure 1.
Pilot Tone: A pilot tone (PT) signal was created by a prototype set-up: The signal was generated by a commercial synthesizer (Hewlett Packard) connected to an additional inhouse-built non-resonant coil fixed to the bore of the scanner. The frequency was set such that the signal was recorded in the two-fold oversampled readout signal of the MR-data. During respiration the intensity of the received PT signal varied, which provided a continuous motion surrogate for each readout.
Respiratory Belt (RB): Two respiratory belt transducers (Biopac Systems, Inc) were fixed to the subjects around the chest and abdomen. The change of circumference of the body during the breathing cycle was measured with an internal air pressure system.
MR-navigator: This commonly used MR-navigator was obtained by tracking the motion of the dome of the right hemi-diaphragm. The motion of the diaphragm relates closely to the motion of the heart.2
Calibration scan: Cardiac, ECG-triggered, dynamic measurements were performed at 3T (MAGNETOM Verio, Siemens Healthcare, Germany) on 3 volunteers (2 female, age 30 ± 2 years). For each cardiac cycle a coronal and sagittal 2D-image were acquired to capture the 3D-motion of the heart with an inhouse-modified FLASH sequence, TE=1.4ms, TR=2.4ms, FOV=300x300mm2 and voxel size=1.56x1.56x8mm3. The calibration scan was carried out for 60s.
Registration + Linear correlation: To determine translation parameters in foot-head (FH), anterior-posterior (AP) and right-left (RL) plane, a region of interest around the heart was chosen manually for both coronal and sagittal view. A normalized cross-correlation function was used to determine the translational breathing motion of the heart in 3D. The surrogate signals were then calibrated to the determined translation using a linear model.
Assessment of temporal stability: To assess the temporal stability of the surrogate signals, the same scan used for calibration was carried out for additional 130s. The motion predictions of the surrogate signals based on the 60s calibration were then compared to the heart motion determined during these additional 130s.
3D high-resolution cardiac scan: A 3D FLASH cardiac-triggered scan with TE=4.1ms, TR=7.3ms, flip angle=12°, FOV=300x244x32mm3 and voxel size=1.56x1.56x2mm3 was obtained during free-breathing. A MR-navigator was obtained for each cardiac cycle but was not used for gating.
Surrogate-based motion correction: Based on the calibration step before, the heart motion for each readout was calculated using the surrogate signals. Motion correction was done prior to offline image reconstruction with Matlab by applying a phase shift to the k-space data, using all MR-raw-data.
Results and Discussion
Conclusion
We have compared the correlation between MR-navigator, PT, RB and the registered cardiac motion due to breathing and we have demonstrated the feasibility to use these surrogate signals for motion correction with high temporal resolution. PT and RB are independent of the MR-data acquisition and can therefore be combined with any MR-scan, making this a very flexible motion-correction approach.Acknowledgements
No acknowledgement found.References
1Scott A D, Keegan J, Firmin, David N. Motion in Cardiovascular MR Imaging. Radiology: Volume 250: Number 2 – February 2009.
2McClelland J R, Hawkes D J, Schaeffter T, King A P. Respiratory motion models: A review. Medical Image Analysis 17, p. 19-42, 2013.
3Aitken A P, Henningsson M, Botnar RM, Schaeffter T, Prieto C. 100% Efficient three-dimensional coronary MR angiography with two-dimensional beat-to-beat translational and bin-to-bin affine motion correction. Magn Reson Med. 2015;74:756–764.
4Luo J, Addy N O, Ingle R R, et al. Nonrigid Motion Correction With 3D Image-Based Navigators for Coronary MR Angiography. Magn Reson Med. 2017;77:1884–1893.
5Schmidt J F M, Buehrer M, Boesiger P, Kozerke S. Nonrigid retrospective respiratory motion correction in whole-heart coronary MRA. Magn Reson Med. 2011;66:1541–1549.
6Bhat H, Ge L, Nielles-Vallespin S, Zuehlsdorff S, Li D. 3D radial sampling and 3D affine transform-based respiratory motion correction technique for free-breathing whole-heart coronary MRA with 100% imaging efficiency. Magn Reson Med. 2011;65:1269–1277.
7Speier S, et al. PT-Nav: A Novel Respiratory Navigation Method for Continuous Acquisition Based on Modulation of a Pilot Tone in the MR-Receiver. ESMRMB 129:97-98, 2015. Doi: 10.1007/s10334-015-0487-2.
Figures