4108
Tracking Respiratory Motion Throughout Arbitrary MRI Sequences via Pilot Tone Navigation1Radiology, NYU School of Medicine, New York, NY, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Siemens Medical Solutions, New York, NY, United States
Synopsis
In this work, we demonstrate a flexible approach to track respiratory motion throughout arbitrary MRI sequences without requiring any additional patient setup time or sequence modification. A reference RF signal, which has previously been referred to as the “pilot tone” (PT), is tracked throughout MR imaging, and its amplitude modulation provides information about respiratory motion. Specifically, we demonstrate continuous tracking of respiratory motion throughout multi-echo and multi-shot sequences on a human volunteer via PT navigation.
Introduction
Since the introduction of simultaneous PET-MR, there have been several efforts to utilize the MR data that is acquired concurrently with PET for motion correction purposes [1,2]. One necessary component to most motion-correction models is continuous monitoring of respiration via a surrogate signal, such as superior/inferior displacement of the diaphragm. Some “self-gated” MRI sequences provide this information inherently [3]; however, most clinical sequences do not fall into this category. Therefore, external devices such as respiratory bellows have been used to track motion across arbitrary sequences. These devices have limitations due to their physical properties and require additional patient setup time, which is a major impediment to their routine clinical use. Previously, the pilot tone (PT) was introduced [4] as an alternative motion-tracking mechanism which is compatible with arbitrary MRI sequences and does not require additional patient setup time. An RF transmitter driven by an external generator produces a fixed-frequency reference signal; its amplitude modulation over time correlates with respiratory motion. In this study, we demonstrate the ability to continuously monitor respiratory motion throughout multi-echo and multi-shot sequences with different trajectories using the PT.Methods
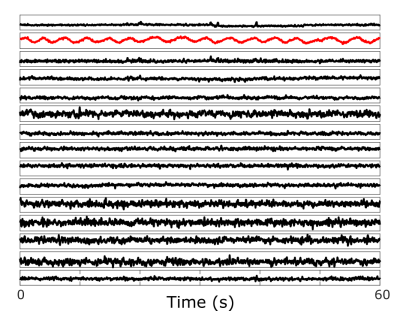
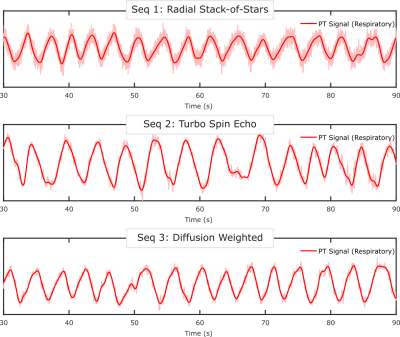
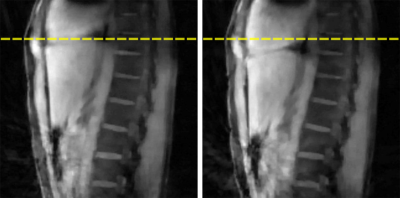
All data were acquired on a Siemens BIOGRAPH mMR (Siemens Healthcare, Erlangen, Germany) using the body coil array in combination with the spine coil and a 2cm surface transmit coil driven by an external signal generator. The coil was mounted on the wall of the scanner room, and its frequency was adjusted for each sequence so that the PT signal was outside the bandwidth of the subjects’ MRI signal. Oversampling of the readout signal provided the required bandwidth to capture the reference signal from the PT. Three sequences were used to highlight the ability of the PT to monitor respiratory motion. The first MRI sequence was an in-house modified prototype 3D stack-of-stars FLASH sequence (TR/TE=9/5.29ms, BW=490Hz/pixel, 88x4.5mm slices, 1000 radial views, 1.56mm in-plane resolution) with a golden-angle (GA) acquisition. Next, an in-house 2D turbo spin echo (TSE) sequence was acquired (TR=1s, BW=521Hz/Pixel, 32x4mm slices, 1.09mm in-plane resolution). Finally, an unmodified Siemens 2D EPI diffusion-weighted sequence was acquired (TR=7.8s, BW=2170Hz/Pixel, 45x6mm slices, 1.37mm in-plane resolution). All data processing and reconstruction was performed offline using MATLAB (MathWorks, Natick, MA). The PT signal was detected and separated from the raw MRI data using a peak detection algorithm in the frequency domain. The second-order blind identification (SOBI) algorithm [5] was applied to the PT amplitude signals from the separate coil channels to isolate the respiratory motion signal. The relevant signal component was automatically selected (Figure 2) by considering the power spectral density within the frequency range of [0.1Hz – 0.5Hz]. MRI data from the first GA stack-of-stars sequence were reconstructed into 10 respiratory-state images (Figure 4). This process was repeated for each subject.Results
Figure 2 depicts the signal components that are obtained after applying SOBI to the PT amplitude signals from the different receive coil channels. The respiratory component is isolated (red) and identified successfully. Figure 2 depicts the data from the diffusion-weighted sequence, but similar results are obtained from all sequences. The respiratory gating signals obtained from all sequences are shown in Figure 3. The motion state images obtained from the golden-angle stack-of-stars sequence validate the respiratory gating signal obtained from the PT during that acquisition. Figure 4 depicts the frames corresponding to end-inspiration (left) and end-expiration (right). The movement of the diaphragm between these states is clearly observed.Discussion
In all sequences, the respiratory motion surrogate is successfully extracted from the pilot tone signal. After the pilot tone transmitter was mounted to the wall, the only necessary intervention was manually adjusting the transmit frequency for each MR sequence, so that the pilot tone remains within the receive band without overlapping the imaging signal; however, automating this process is straight-forward. This demonstrated ability to track respiratory motion across multiple MR sequences without modifying them is particularly attractive for PET-MR applications where motion correction is desired. This could allow for also motion-correcting PET data that is acquired simultaneously with routine clinical MRI protocols. Since the PT-based motion tracking signal is recorded by the standard MR receive coils, the temporal resolution of this gating signal is related to the repetition time of the sequence. For sequences with long magnetization preparation modules (e.g., Inversion Recovery), the use of additional readouts could be an effective means to provide the needed temporal resolution.Conclusion
We have demonstrated the ability to track respiratory motion throughout three arbitrary MRI sequences using the pilot tone navigator. This approach generally requires no modification of imaging sequences or additional patient setup time.Acknowledgements
No acknowledgement found.References
[1] T. Vahle et al., ISMRM 2017, #3897.
[2] R. Grimm et al., Med. Image Anal, 2015, 19: 110-20.
[3] Feng, Li et al. “XD-GRASP: Golden-Angle Radial MRI with Reconstruction of Extra Motion-State Dimensions Using Compressed Sensing.” Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 75.2 (2016): 775–788. Web.
[4] P. Speier et al., Proc. ESMRMB 2015, 129: 97-98.
[5] Belouchrani, A. et al. “A Blind Source Separation Technique Using Second-Order Statistics.” IEEE transactions on signal processing: a publication of the IEEE Signal Processing Society 45.2 (1997): 434–444.
Figures