4106
Improved non-rigid motion suppression for free-breathing PROPELLER with adaptively weighted blade combination1AllTech Medical Systems, Chengdu, China, 2Alltech Medical System America, Solon, OH, United States
Synopsis
PROPELLER has been applied to suppress respiratory motion for free-breathing abdomen imaging but the results are often unsatisfactory with existing weighting mechanisms. In this study, a novel adaptive weighting method is proposed to maximize the respiratory motion suppression without using a fallible reference blade. First, mutual information is used to measure the motion correlation across different blades. Second, principal components analysis is applied to adaptively reject/keep the acquired data by assigning proper weights to all blades. Our experiments show that the proposed method can provide abdominal images with less blurring and less partial volume artifacts compare to the conventional PROPELLER.
Purpose
To improve the respiratory motion suppression for free breathing PROPELLER.Introduction
PROPELLER is a motion suppression technique that can effectively correct the rotation and translation movement during scan1-5. For respiratory motion, it is more complex and difficult for model-based analysis. However, it can still be largely suppressed by assigning proper weights to each blade6-8. Eqn (1) is conventionally used to calculate the PROPELLER weights1,2. $$C_{i}=\left | \sum D_{Ref}D_{i}^{*} \right |\qquad\qquad(1)$$Where $$$D_{i}$$$ is the $$$i^{th}$$$ blade data gridded onto the central circular k-space, and $$$D_{Ref}$$$ is the reference via either averaging1,2 or grouping9. This equation can efficiently detect in-plane translation and rotation but cannot accurately reflect the correlation of blades with non-rigid respiratory motion. To solve this problem, we propose to use mutual information, which has been proven as an accurate measurement of image correlation for both rigid and non-rigid motion11-13, to calculate the weights of different blades. In addition, to avoid explicit $$$D_{Ref}$$$ selection that can be error-prone via averaging or grouping when large respiratory motion exists, principal components analysis is applied to adaptively reject/keep the acquired data.
Theory
In PROPELLER, the acquired low spatial image $$$M_{i}$$$ from the $$$i^{th}$$$ blade can be considered as two parts: a desired stochastic signal process $$$M$$$, plus an undesired stochastic noise process (respiratory motion) $$$\delta_{i}$$$ $$M_{i}=M+\delta_{i}\qquad\qquad(2)$$The optimization target is to find a weighting vector that maximizes the signal $$$M$$$ over random motion $$$\delta_{i}$$$.$$M=\sum w_{i}M_{i}\qquad\qquad(3)$$Here, the mutual information of blade i and j is used to measure their correlation, which can be equivalently expressed as$$I(M_{i},M_{j})=H(M_{i})+H(M_{j})-H(M_{i},M_{j})\qquad\qquad(4)$$ where $$$H(M_{i})$$$, $$$H(M_{j})$$$ are the marginal entropies11, and $$$H(M_{i},M_{j})$$$ is the joint entropy of $$$M_{i}$$$ and $$$M_{j}$$$11. Let $$$R_{ij}=I(M_{i},M_{j})$$$, then $$$R$$$ is the correlation matrix, which can be decomposed into linearly independent components by principal components analysis14,15. The main components represent the amount of the shared information of different blades, i.e., the weights. The singular vector $$$γ$$$ with the largest singular value solved by singular-value decomposition (SVD) can be used to approximate the principal components. Finally, $$$γ$$$ was spanned via eqn (5)1 to balance the tradeoff between the rejection of bad data and the smoothing effect.$$w_{i}=(a+(1-a)\frac{\gamma _{i} - \gamma_{min}}{\gamma _{max} - \gamma_{min}})^p \qquad\qquad(5)$$Where, $$$\gamma_{min}$$$ and $$$\gamma_{max}$$$ are the minimal and maximal of $$$\gamma$$$ , a and p are tradeoff factors.Methods
Acquisition: All experiments were performed on a 1.5T whole-body scanner (Centauri, Alltech Medical Systems). Eight healthy volunteers (5 male and 3 female; mean age= 32) were scanned with free-breathing. T2 FSE PROPELLER sequence with golden angle rotation10 was used and the acquisition parameters were: FOV 400x400mm2, Matrix size 256x256, thickness/gap 6/1.5mm, 24 slices, TR/TE 5400/102ms, ETL 26, 24 blades with 44 lines/blade in-plane GRAPPA R factor 2, 8 ACS lines/blade. Total scan time was 2min20sec.
Reconstruction: The reconstruction was implemented using MATLAB on a PC with Intel i7@3.6GHz and 16G RAM. The raw PROPELLER data first went through the phase correction, rotation correction and translation correction1,2. Then the adaptive weighting for each blade was calculated before the final blade combination. $$$M_{i}$$$ reconstructed from the center k-space of each blade was zero-padded to 128x128, and compressed to 64 gray-scale-levels for mutual information calculation. a=0.1, p=2 for eqn (5) were set empirically. The total reconstruction time was 305sec and 303sec for the proposed method and conventional PROPELLER respectively.
Results and Discussion
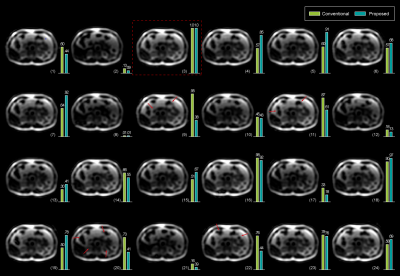
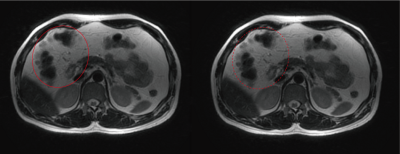
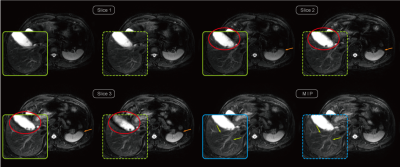
Exemplary Low spatial images reconstructed from the center k-space of different blades are shown in Figure 1. Their corresponding weighting values via both the conventional and proposed methods are also listed respectively. The proposed method provides more reasonable weight values than the conventional method. Final reconstructed results after blade combination are shown in Figure 2. The image with the proposed method exhibits less blurring around some intestinal organs. Figures 3 demonstrates three adjacent slices with CHESS fat suppression, less blurring and partial volume artifacts can be observed on the images combined using the proposed adaptive weights. More ducts and slow-flow vessels can also be identified on the maximum intensity projection (MIP) image with the proposed method. The computation time of mutual information is negligible due to the reduced size and gray-scale-levels of $$$M_{i}$$$, resulting in no significant increase of total reconstruction time.Conclusions
An adaptively weighted blade combination method for free-breathing PROPELLER based on mutual information correlation and PCA has been developed and validated in volunteer scans. The proposed method can improve the non-rigid motion suppression for free-breathing abdominal imaging without significant increase of reconstruction time.Acknowledgements
No acknowledgement found.References
- Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med 1999; 42:963–969.
- Pipe JG, Gibbs WN, Li Z, et al. Revised Motion Estimation Algorithm for PROPELLER MRI. Magn Reson Med 2014; 72:430–437.
- Forbes KP, Pipe JG, Bird CR, et al. PROPELLER MRI: clinical testing of a novel technique for quantification and compensation of head motion. J Magn Reson Imag 2001; 14:215–222.
- Pipe JG. Improved in-plane motion correction for PROPELLER MRI. Proc. Intl. Soc. Mag. Reson. Med Scotland, 2001. (743).
- Holmes JH, Beatty PJ, Rowley HA, et al. Improved motion correction capabilities for fast spin echo T1 FLAIR propeller using non-cartesian external calibration data driven parallel imaging. Magn Reson Med 2012; 68:1856–1865.
- Deng J, Larson AC. Modified PROPELLER approach for T2-mapping of the abdomen. Magn Reson Med 2009; 61:1269–1278.
- Hirokawa Y, Isoda H, Maetani YS, et al. Evaluation of Motion Correction Effect and Image Quality With the Periodically Rotated Overlapping Parallel Lines With Enhanced Reconstruction (PROPELLER) (BLADE) and Parallel Imaging Acquisition Technique in the Upper Abdomen. J Magn Reson Imag 2008; 28:957–962.
- Turley DC, Schär M, and Pipe JG. Improved Reconstruction of Free-Breathing Abdominal PROPELLER MRI: A Preliminary Study. Proc. Intl. Soc. Mag. Reson. Med. 22, 2014.
- Liu Z, Zhang Z and Ying K.Improved Motion Correction in PROPELLER by Using Grouped Blades as Reference. J Magn Reson Imag 2014;39:700–707.
- Nehrke K, Börnert P, Yperen GH, et al. Golden Angle PROPELLER MRI. Proc. Intl. Soc. Mag. Reson. Med. 15, 2007. (1729)
- Maes F, Collignon A, Vandermeulen D, et al, Multimodality image registration by maximization of mutual information. IEEE Transactions on Medical Imaging 16 (1997), 187–198.
- Maes F, Vandermeulen D and Suetens P, Comparative evaluation of multiresolution optimization strategies for multimodality image registration by maximization of mutual information. Medical Image Analysis 1999; Vol(4): 373–386.
- Pluim JPW, Maintz JBA and Viergever MA, Mutual information based registration of medical images: a survey. IEEE Transactions on Medical Imaging 2003; Vol 22(8): 986-1004.
- Jolliffe, IT. Principal Component Analysis, second Springer-Verlag. 2002; ISBN 978-0-387-95442-4.15. Walsh DO, Gmitro AF and Marcellin MW. Adaptive Reconstruction of Phased Array MR Imagery. Magn Reson Med 2000; 43:682–690.
Figures