4100
Motion robust Magnetic Resonance Fingerprinting using Echo-Planar Imaging and intensity based motion correction1Computer Assisted Clinical Medicine, University Medical Center Mannheim, Heidelberg University, Heidelberg, Germany, 2Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN, United States, 3Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Synopsis
Quantitative magnetic resonance imaging (MRI) enables the quantification of biomarkers and the detection of tissue changes. Recently, various magnetic resonance fingerprinting (MRF) sequences were proposed to enable simultaneous quantification of T1 and T2/T2*relaxation times. In this study, we demonstrate motion sensitivity of the original MRF and MRF-EPI. Intensity-based image registration is used in six healthy subjects to eliminate motion artifacts in MRF-EPI. The effectiveness is demonstrated by a significant improvement in the Dice coefficient. As the algorithm is independent of patient and slice, it facilitates motion robust acquisition of parameters maps with MRF ready for clinical use.
Introduction
Patient motion is one of the main causes of artifacts and reduced image quality in clinical MRI. A novel technique known as magnetic resonance fingerprinting (MRF) is a promising method for rapid quantitative imaging1, acquiring numerous baseline images in rapid succession while contrast weighting is varied using pseudo-randomized sequence parameters. However, long scan times compared to qualitative measurements commonly promote motion-sensitivity in quantitative imaging. In this study, the motion sensitivity of two MRF approaches, based on bSSFP spiral imaging1 and FLASH-EPI2 is analyzed by simulations. This data was corroborated by in-vivo measurements using MRF-EPI. Furthermore, a retrospective motion correction algorithm is proposed to alleviate residual motion sensitivity in the measurements.Mehtods
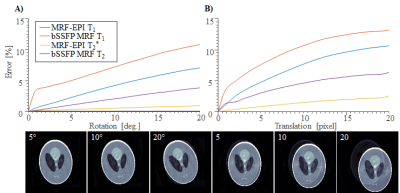
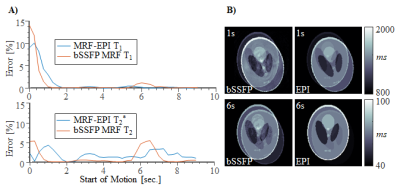
NUMERICAL SIMULATION: Original MRF and MRF-EPI were simulated in a Shepp-Logan phantom using Bloch-equations, with 𝑇1 and 𝑇2/𝑇2*(𝑇2 for original MRF and 𝑇2* for MRF-EPI) in the in-vivo range. Sequence parameters of original MRF and MRF-EPI were chosen as previously described1,2. Motion was induced in two sets of experiments by corrupting 1 second worth of images: 1) Variable amplitude (0°-20° rotation, 0-20 pixel translation) at the beginning of the measurement. 2) Fixed motion amplitude (14º rotation and 10 pixel translation), with varying time of onset of the motion. The resulting 𝑇1 and 𝑇2/𝑇2*-maps from the motion corrupted fingerprints were determined and the mean deviation from the ground truth was calculated.
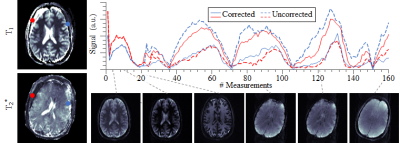
IN-VIVO EXPERIMENTS: In-vivo measurements of 6 healthy volunteers (4 male, 27±3 years) were acquired with MRF-EPI on a 3 T whole-body scanner (Siemens Trio) using a 32-channel head-array-coil, with following sequence parameters: TE/TR=17-78ms/80-755ms, flip angle=4-58°, FOV=220x220x140mm3, voxel size=1.7x1.7x5.0mm3, band-width=1136Hz/pixel, partial-Fourier=5/8, GRAPPA rate=3, acquisition time slice=10s, total number of baseline images=160. To demonstrate motion sensitivity and to validate correction, subjects were instructed to turn their heads by 20 degrees 2 seconds after the scan start. Subsequently, a reference measurement without motion was acquired for each test person.
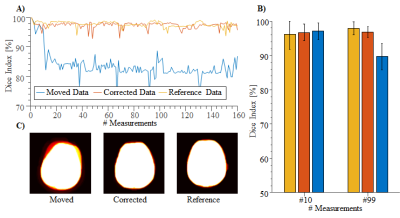
MOTION CORRECTION: Motion correction was performed using rigid geometric transformation based on mutual information with a 1+1 evolution strategy with following parameters: maximum iteration=1000, initial radius=0.0156, epsilon=1.5·10-6, growth factor=1.05. The first image was selected as reference due to the highest SNR. The motion correction is evaluated by means of visual assessment of parameter maps and quantitative assessment of Dice indices. The scan series are statistically compared using Student’s t-test on the log-transformed indices of baseline image 10 (before motion) and 99 (after motion).
Results
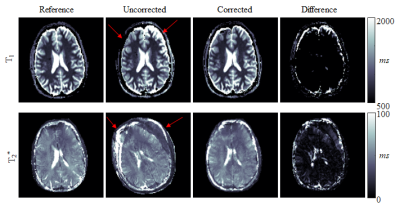
Simulation results show high motion sensitivity of both MRF methods, even to small displacements (Fig. 1). 𝑇1 quantification is particularly susceptible to motion in the early parts of the scan, while 𝑇2 and 𝑇2* are more resilient to motion with a uniform sensitivity profile throughout the measurement (Fig. 2). Artifacts such as blurred edges are clearly visible in the simulated parameter maps generated from motion corrupted data (Fig. 1,2). This can be confirmed by in-vivo measurements. Both, 𝑇1 and 𝑇2* parameter maps show artifacts such as ghosting and fuzziness (Fig. 4). However, image artifacts are successfully mitigated with the proposed intensity based co-registration method (Fig. 4). The correction significantly increases the Dice index from 90% in the presence of motion to 97% after correction (p < 0.002 Fig. 5), which is comparable to data without motion (p = 0.264 Fig 5).
Discussion
In this work, it was shown that MRF exhibits residual motion sensitivity, which was successfully mitigated in MRF-EPI using retrospective motion correction. Simulation and in-vivo results show that a movement causes artifacts including ghosting and blurring. 𝑇1 quantification is particularly susceptible to motion in the early parts of the scan because of the dominant longitudinal magnetization in this period. Due to the increased SNR, the baseline images of the MRF-EPI are well suited for motion correction. The correction works independently of slice and subject. For original MRF based on highly undersampled spirals, undersampling artifacts might cause image registration to fail. Here, sliding window reconstruction might be required to employ the proposed motion correction technique. Motion correction in MRF, is particularly challenging due to a wide range of observed imaging contrasts, commonly causing intensity based co-registration schemes to fail. Mutual information was used to overcome this problem in the proposed approach yielding consistent registration Performance. While the proposed method can only be applied to correct for inter-image motion, residual intra-image motion remains a limitation of the proposed post-processing. However, highly accelerated EPI readouts yield rapid imaging, which greatly limits the patient motion during the readout.Conclusion
Although residual motion sensitivity is present in MRF, our data indicates, that MRF-EPI can be reliably performed in the presence of motion, by employing retrospective image registration on the baseline images. This fosters motion robustness, which can benefit the clinical use of quantitative MRI.
Acknowledgements
No acknowledgement found.References
1. Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, et al. Magnetic resonance fingerprinting. Nature. 2013 Mar 14;495(7440):187–92.
2. Rieger B, Zimmer F, Zapp J, Weingärtner S, Schad LR. Magnetic resonance fingerprinting using echo-planar imaging: Joint quantification of T1 and T2∗ relaxation times. Magn Reson Med. 2016 Dec 1;n/a-n/a.
Figures