4099
Ultra-High-Resolution Dental MR Imaging Using an Ultra-Short-TE Sequence with Prospective Motion Correction1Department of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 3Department of Radiology, University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 4Department of Radiology and Neuroradiology, Section Biomedical Imaging, MOIN CC University Medical Center Schleswig-Holstein, University of Kiel, Kiel, Germany
Synopsis
High-resolution dental MR Imaging with clear boundaries is of interest for clinical applications e.g. for tooth implant planning. Due to the fast signal decay of the dental tissues, 3D ultra-short-TE (3D-UTE) sequences are employed, which combine rapid center-out half-projection readouts with a phyllotaxis ordering on the surface of
Introduction
3D UTE (ultra-short TE) sequence can be effectively used to image tissues with short T2* relaxation times as seen in teeth, menisci or tendons. Unlike a conventional radial trajectory, the acquisition in 3T-UTE starts with the ramp-up of the readout gradient immediately after the excitation. Conventional radial imaging shows a high robustness against motion, which can be further enhanced by retrospective correction methods [1]. However, half-projection readouts of 3D-UTE present a challenge to the established post-processing techniques. Due to relatively long measurement times required for high-resolution dental MRI, motion artifacts as blurring and streaking limit the potential clinical applications. Prospective motion correction (PMC) with external motion tracking has been successfully utilized to achieve a very high spatial resolution in vivo [2, 3], but primarily in combination with Cartesian k-space sampling trajectories.
In this work, we employ projection imaging with a phyllotaxis encoding ordering [4] as a way to arrange samples on the surface of a sphere. To optimize the encoding we generated two trajectories: single-spiral (SS) and multi-spiral (MS) spherical sampling. They were compared in phantom experiments against different motion types. We then apply the MS version of the sequence with PMC to ultra-high-resolution dental MR imaging in vivo.
Methods
Experiments were conducted on a 3T MAGNETOM Prisma system (Siemens Healthcare, Erlangen, Germany). An in-bore optical system equipped with a Moiré pattern marker (MPT, Metria Innovation Inc. Milwaukee, USA) was used to track motion with 6 degrees-of-freedom at a frame rate of 85Hz [3]. Both SS and MS readout orderings follow a prototype phyllotactic spiral [4] to achieve isotropic sampling on the surface of the sphere. The gradient rotation matrix is thereby manually updated. XPACE library [5] was used for slice position updates prior to the excitation and was extended to allow for custom modifications of the rotation matrices within the sequence.
In the phantom measurements, three motion trajectories (no motion, rotation along Y direction by 3°, translation along X and Z direction by 3 mm) were tested with 1 mm resolution for SS and MS (N=10) sampling using a TX/RX coil.
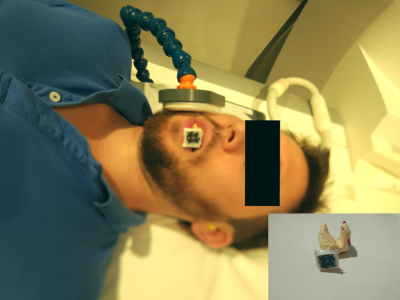
In vivo, MS sampling was used with the following parameters: TE 50 µs and 3050 µs; TR 8 ms; bandwidth 840 Hz/Pixel; resolution 0.35 mm isotropic; field of view 67 mm, flip angle 10°; 36,870 spokes and 10 spiral spheres for each TE. In order to enhance the MR signal, a homemade inductively-coupled intraoral receive coil [6] and a standard 4 cm single-loop coil were used [see Fig. 1]. The intraoral coil was covered with dental-grade putty for fixation and better visualization of the teeth surface. The MPT marker was attached to the intraoral coil for tracking the position of the subject.
Kaiser-Bessel gridding with Hanning filter was used for offline reconstruction with MATLAB (2016a, The MathWorks, Natick, MA, USA). The same protocol was run twice, with and without PMC. In both cases, the subject was asked to keep as still as possible. As a reference an ex vivo MR image of a human jaw is presented.
Results & Discussion
Fig. 2 displays a comparison of SS and MS sampling with different motion patterns, especially along the slow direction of the trajectory. In general, SS shows stronger blurring artifacts, whereas MS displays stronger streaking artifacts. This is due to the fact that MS has a shorter delay in X direction, but stronger motion-induced phase inconsistencies between the neighboring spokes. In general, MS shows a higher robustness against motion with less blurring. Thus, as a target of this study was high-resolution imaging, we have chosen the MS trajectory for in vivo experiments.
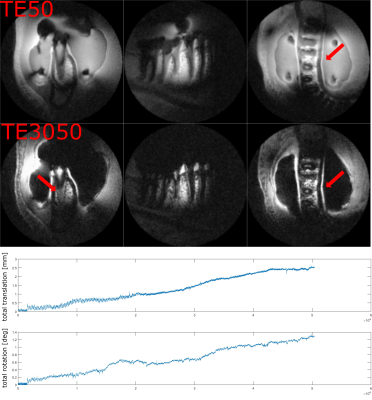
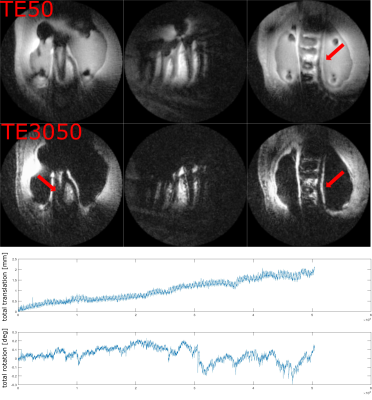
Fig. 3 and 4 show dual-echo dental imaging results with and without PMC. Motion information is presented in corresponding plots below. As the amount of motion in both scans is comparable, less blurring and streaking artifacts with PMC (red arrow) result in clearer boundaries.
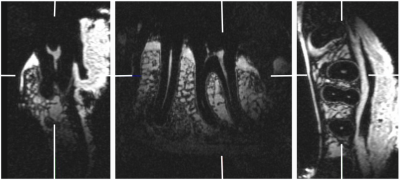
Fig. 5 displays dental MR image of ex vivo specimen with Cartesian sampling. As seen, the image quality in vivo with PMC is comparable with the ex vivo specimen.
Of note is the unusual appearance of the motion artifacts in Fig. 4. In contrast to traditional Cartesian imaging, motion-affected images here show no ghosting, but dominant blurring and streaking instead. As seen in Fig. 3, these kinds of motion artifacts can effectively be avoided by PMC. Compared to our previous results where SS 3D-imaging with PMC was used [7], dental imaging with MS and PMC shows even higher image quality.
We hypothesize that the remaining imperfections originate from interactions between the trajectory imperfections and motion. They will be addressed in future by combined prospective-retrospective correction taking into account gradient delays and other gradient trajectory deviations.
Acknowledgements
This work was funded in part by NIH grant 2R01DA021146 and Chinese Scholarship Council.References
1. Shankaranarayanan A, Wendt M, Lewin J S, et al. Two‐step navigatorless correction algorithm for radial k‐space MRI acquisitions. Magnetic resonance in medicine. 2001;45(2):277-288.
2. Stucht D, Danishad K A, Schulze P, et al. Highest resolution in vivo human brain MRI using prospective motion correction. PloS one. 2015;10(7):e0133921.
3. Lüsebrink F, Sciarra A, Mattern H, et al. T1-weighted in vivo human whole brain MRI dataset with an ultrahigh isotropic resolution of 250 μm. Scientific Data. 2017;4:170032.
4. Piccini D, Littmann A, Nielles‐Vallespin S, et al. Spiral phyllotaxis: the natural way to construct a 3D radial trajectory in MRI. Magnetic resonance in medicine. 2011;66(4):1049-1056.
5. Zaitsev M, Dold C, Sakas G, et al. Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. Neuroimage. 2006;31(3):1038-1050.
6. Ludwig U, Eisenbeiss A K, Scheifele C, et al. Dental MRI using wireless intraoral coils. Scientific reports. 2016;6:23301.
7. Gao X, Fischer J, Amrein P, et al. Ultra-short TE Imaging with Prospective Motion Correction. ISMRM Workshop on Motion Correction in MRI & MRS. South Africa. 2017.
Figures