4095
Deep learning-based real time MRI1Department of Radiological Sciences, University of California, Los Angeles, los angeles, CA, United States, 2Biomedical Physics Interdepartmental Program, University of California, Los Angeles, los angeles, CA, United States, 3Department of Bioengineering, University of California, Los Angeles, los angeles, CA, United States
Synopsis
In this work, we use the dilated U-net to reconstruct the dynamic free breathing cine images from regular under sampled raw data. Also, we consider different under sampling rate to determine maximum achievable rate. Moreover, we modify the acquisition procedure of sequence to show the possibility of prospective dynamic imaging. The proposed method is capable of reconstructing high quality real time 2D cardiac images with up to 6X acceleration with excellent image quality and minimal latency of only 6ms on a typical workstation.
Introduction
Real time MRI is a promising imaging modality for MRI-guided interventions. Due to the need for immediate feedback from the images, it is important to acquire the MR signal and reconstruct the image with minimal latency. Recent advances in deep neural networks enable new possibilities for real time imaging reconstruction due to its much shorter image reconstruction time compared to traditional CS reconstructions 1,2,3,4. In this work, we propose to use a dilated U-net for real time image reconstruction and to demonstrate its feasibility in prospectively under-sampled real time cardiac cine MRI.Methods
Convolutional U-net5 used in previous studies to reconstruct the MR images consists of a contraction followed by an expansion. In conventional U-net, all the layers in contracting path is convolution layer which generally follows by ReLU and subsampled by max-pooling. We replace the convolution-layers producing the feature maps in the contracting path of the conventional U-Net with dilated convolutions to expand receptive fields. Our network architecture is shown in Fig.1 (b). Free-breathing fully sampled real-time cardiac cine images were acquired from five healthy volunteers. The dataset from each volunteer had 200 temporal frames of different sections. The first three volunteers’ data were used in training the neural network (1300 images in total), and the fourth volunteer was used as a validation set (500 images). The fifth volunteer data (200 images) was used to test the network by under-sampling the data and feeding it into the network. Data preparation is shown in Fig.1 (a). To train the network, all weights were initialized by random values from a normal distribution with zero mean and a 0.005 standard deviation. An important aspect of the training process is to decide on the loss function. We compared three loss functions for training our network: L2, L1, and dissimilarity loss function 6 (DSSIM). The loss functions were minimized by using the RMSPropOptimizer with a learning rate of 0.001. Different under-sampling rates were evaluated to determine maximum achievable rate. In addition, we modify a real time MRI sequence to acquire under-sampled k-space from a separate volunteer to show the feasibility of using our technique for reconstructing prospectively under-sampled real time MRI.Results
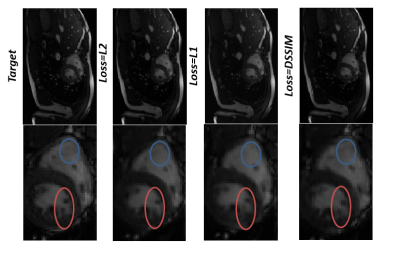
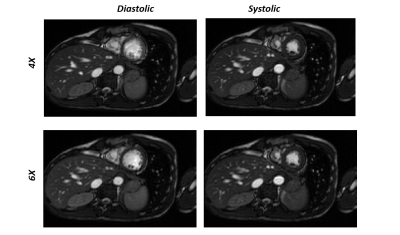
Reconstruction results for diastolic phase is shown in Fig.2 for different under-sampling rate and different loss functions. Statistical Analyses for peak SNR (PSNR) and SSIM is summarized in Table.1(a&b). In Fig.3, the effects of different loss functions are presented, which focuses on segments of the right ventricle (blue circle) and left ventricle (orange circle) to show their differences, although subtle. Based on our preliminary results, for regular under sampling pattern, 8-fold acceleration is the maximum achievable rate without significantly compromising reconstruction quality. However, implementing prior knowledge or changes the sampling pattern to irregular under-sampling pattern may potentially increase the achievable acceleration rate. For our prospective study, systolic and diastolic reconstructed frames for 4X and 6X acceleration are shown in Fig.4. The image reconstruction time was 6 ms for each image, which is clearly within acceptable range of latency for real time applications.Discussion and Conclusions
L1 loss function in contrast to L2 does not over penalize larger error. As shown in Fig.3, for L1, splotchy artifacts are less significant than L2, but the L2 loss function preserves edges better than L1. However the DSSIM loss function is roughly closest to target image. Receptive fields of convolutional neural network plays an important role in image reconstruction. While simple convolution increase the receptive fields linearly, dilated convolution increase the receptive fields exponentially. In other words, dilated convolution can decrease the number of layers (depth) which is required to train a network. Therefore, it is computationally efficient and required less parameter to be learned. Deep Convolutional Neural Network in reconstruction task is only applicable to data with fixed matrix size. For different matrix size, another network should be trained or data must be resized in k-space domain before feeding to the network.
All in All, Deep learning-based approaches can greatly shorten the MR acquisition time and reduce the reconstruction latency, making it suitable for real time imaging. The proposed method is capable of reconstructing high quality real time 2D cardiac images with up to 6X acceleration with excellent image quality and minimal latency of only 6ms on a typical workstation. Future study is needed to optimize the network parameters and test it on more clinical datasets.
Acknowledgements
No acknowledgement found.References
1] C. M. Sandino and J. Y. Cheng, “Deep convolutional neural networks for accelerated dynamic magnetic resonance imaging.”
[2] C. M. Hyun, H. P. Kim, S. M. Lee, S. Lee, J. K. Seo, S. Korea, and S. Korea, “Deep learning for undersampled MRI reconstruction,” no. 1, pp. 1–11.
[3] J. Schlemper, J. Caballero, J. V Hajnal, A. Price, and D. Rueckert, “A Deep Cascade of Convolutional Neural Networks for Dynamic MR Image Reconstruction,” pp. 1–10.
[4] D. Lee, J. Yoo, J. C. Ye, and C. V Mar, “Deep artifact learning for compressed sensing and parallel MRI.”
[5] O. Ronneberger, P. Fischer, and T. Brox, “U-Net : Convolutional Networks for Biomedical,” pp. 234–241, 2015.
[6] H. Zhao, O. Gallo, I. Frosio, and J. Kautz, “arXiv : 1511 . 08861v2 [ cs . CV ] 14 Jun 2016 Loss Functions for Neural Networks for Image.”
Figures
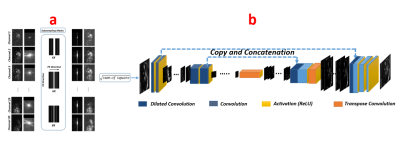
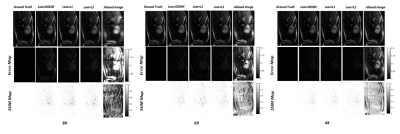
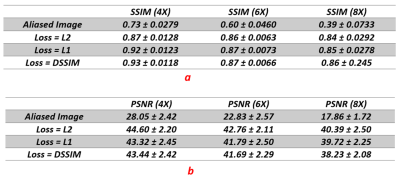
Table.1: (a) mean and standard deviation of Structural Similarity Index over 200 test frames. Average of SSIM map for each test frame were calculated and then reported the mean and standard deviation of these average values. (b): mean and standard deviation of Peak Signal to Noise Ratio over 200 test frames.