4084
Segmented 3D fat navigators for faster brain motion tracking at 7T1LIFMET, EPFL, Lausanne, Switzerland, 2CUBRIC, School of Engineering, Cardiff University, United Kingdom
Synopsis
Fat Navigators have previously demonstrated their utility for high-accuracy motion tracking. We investigate a segmented 3D version of the navigators which allows significant acquisition time reduction while maintaining high motion accuracy.
Introduction
Motion is a major source of artefacts in MRI. It has been shown that highly accelerated 3D fat selective navigators (FatNavs) can quantify and retrospectively correct involuntary motion1 at 7T. At lower field, 3D-EPI is an established approach for a (non-fat selective) vNav2 and EPI versions of FatNavs have also been implemented3,4 using 2D projections. Preliminary work has also demonstrated that EPI-based 3D-FatNavs can also function well at 7T5.
In this work we attempt to combine the speed of EPI with the spatial fidelity of GRE by implementing segmented 3D FatNavs (SegFatNavs) and comparing with standard 3D GRE-based FatNavs at 7T. SegFatNavs are equivalent to a multi-shot 3D-EPI with low EPI-factor. They are faster to acquire than full 3D-GRE and offer a reduced number of excitation pulses in the navigator, further reducing the likelihood of perturbing the main imaging sequence. Adjusting the number of segments in the SegFatNav allows control over the compromise between acquisition time and EPI-like spatial distortions. The SegFatNavs approach may also be interesting at lower field, as the fat selective binomial pulses become significantly longer and reducing the number of excitation pulses can significantly speed up the acquisition. At 7T the EPI artefacts are more severe, and while previous EPI FatNavs has shown encouraging preliminary results, the accuracy of the estimated motion parameters should be compared to the 3D-GRE-based FatNavs.
Methods
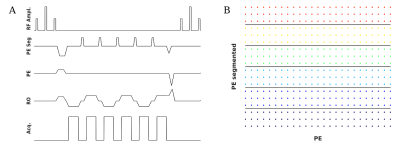
The SegFatNav sequence kernel and the distribution of the segments in k-space are shown in Figure 1. The experiment was performed on a 7T MR scanner (Siemens Healthcare, Germany) using a 32-channel RF coil (Nova Medical Inc.). We scanned one healthy subject, in accordance with the local ethics directives. 70 FatNavs and 70 SegFatNavs were acquired alternately. The scan was repeated for different numbers of segments. For each scan, the subject was asked to reproduce a similar deliberate motion pattern.
The FatNav parameters were 2mm isotropic resolution, 128x128x96 matrix size, ¾ partial Fourier in both phase-encoding directions, 15° flip-angle, under-sampling factor: 4x4. The echo-time of the first echo was always 1.3 ms. The volume acquisition time was 1382 ms for the FatNavs, and the TR for the SegFatNavs was the minimal value achievable by the gradient hardware, leading to volume acquisition times of 884 / 737 / 611 / 575 / 519 / 454 / 440 ms for 2 / 3 / 5 / 6 / 9 / 18 / 25 segments respectively. The GRAPPA calibration data (or ACS lines) for the navigator reconstruction was acquired as a pre-scan (3 s) for each dataset. Coils were combined using root-sum-of-squares. EPI-ghosting correction was performed by applying both a phase ramp (after Fourier transform along the readout direction every other segments) and segment-specific phase factors. The phase ramp and phase factors were determined from a calibration prescan where only readout gradients were played.
FatNavs and SegFatNavs were separately co-registered using SPM to obtain the time-courses of the rigid body motion parameters. The motion parameters from the FatNavs were linearly interpolated to the measurement times of the SegFatNavs before computing the RMSE between the two measures. We interpret this RMSE as an indicator of the relative precision of the segmented navigator approach.
Results and discussion
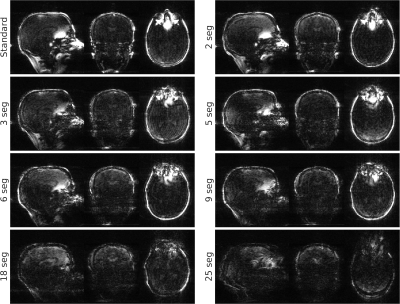
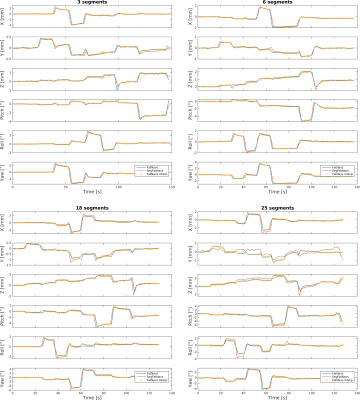
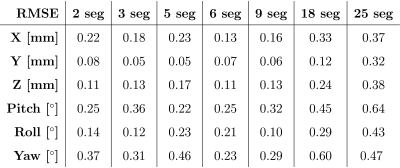
Orthogonal views of the different navigators are shown in Figure 2. Visual inspection shows the reconstruction quality suffers significantly from 18 segments onwards. Examples of (non-interpolated) motion parameter time-courses are presented in Figure 3. Table 1 shows the RMSE between the SegFatNavs and the FatNavs motion estimates. These values also suggest that the motion estimates start to suffer significantly from 18 segments, especially when used for high-resolution imaging motion correction. The 6-segment data shows a significant decrease of acquisition time (~50%) with minimal impact on the estimated motion.
Despite the use of linear interpolation to temporally match the two motion-measures before comparing them, there will remain a slight bias in the RMSE metric due to the acquisitions occurring at different time points. This could be further minimised in future experiments by keeping the motion patterns as smooth as possible6.
Conclusion
While further tweaking of the experimental parameters remains to be investigated, these initial results demonstrate that we can explore the limit at which acquisition speed impacts significantly the precision of the estimated motion at 7T. This work also opens the way for more flexible implementations of 3D Fat Navigators.Acknowledgements
This work was supported by CIBM of the UNIL, UNIGE, HUG, CHUV, EPFL and the Leenaards and Jeantet Foundations, as well as SNSF project number 205321_153564.References
1. Gallichan D, Marques JP, Gruetter R. Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T. Magnetic Resonance in Medicine. 2015;0:1–10.
2. Tisdall MD, Hess AT, Reuter M, Meintjes EM, Fischl B, Van Der Kouwe AJW. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magnetic Resonance in Medicine. 2012;68(2):389–399.
3. Engström M, Mårtensson M, Avventi E, Norbeck O, Skare S. Collapsed fat navigators for brain 3D rigid body motion. Magnetic Resonance Imaging. 2015;33(8):984–991.
4. Skare S, Hartwig A, Mårtensson M, Avventi E, Engström M. Properties of a 2D fat navigator for prospective image domain correction of nodding motion in brain MRI. Magnetic resonance in medicine. 2015;73(3):1110–9.
5. Buur PF, van der Zwaag W, Marques JP, Gallichan D. Fast and flexible 3D-EPI fat navigators for high-resolution brain imaging at 7 Tesla. In: ISMRM. 2016. p. #1870.
6. Gallichan D, Marques JP. Optimizing the acceleration and resolution of three-dimensional fat image navigators for high-resolution motion correction at 7T. Magnetic Resonance in Medicine. 2017;77(2):547–558.
Figures