4080
Fast and Robust: Prospective Motion Correction Combined with Compressed Sensing for 3D Time-of-Flight MRA1Siemens Healthcare GmbH, Erlangen, Germany, 2Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 3Department of Radiology, University Hospital Lausanne (CHUV), Lausanne, Switzerland, 4Signal Processing Laboratory (LTS5), École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland
Synopsis
We present the combination of prospective motion correction and compressed sensing for 3D Time-of-Flight MRA. This enables fast TOF imaging in high isotropic resolution, while preserving image quality and diagnostic value even in the presence of motion. The method was evaluated in five healthy volunteers and image quality was compared to a conventional TOF MRA. Prospective motion compensation successfully enabled robust diagnostic image quality in the highly accelerated scan. The promising results as well as the full integration of the proposed method in a standard clinical scanner enable a comprehensive evaluation in patients in the near future.
Introduction
Fast data acquisition with robust diagnostic image quality is crucial for patients suffering from acute ischemic stroke or intracranial hemorrhages while these patients are not always able to cooperate and hold still. Non-contrast MR Angiography (Time-of-Flight, TOF) is particularly useful in these patients to detect vascular occlusion and/or stenosis1. In recent years, compressed sensing2 has proven to significantly accelerate the data acquisition for TOF MRA, while preserving image quality and diagnostic value3,4. However, image quality is degraded in the presence of uncompensated patient motion during the data acquisition. Prospective motion compensation approaches using optical tracking systems5 have been proposed to maintain diagnostic image quality in case of motion. In this work, we combine compressed sensing with prospective motion correction to facilitate fast and robust TOF imaging at high isotropic resolution. In-vivo experiments were performed in five volunteers and the method was compared to conventional TOF MRA.Methods
For prospective motion correction, an optical camera system (KinetiCor, Honolulu, HI, USA) is mounted on the inside of the scanner bore as described in Zaitsev6. A marker is attached to the subject’s nose between the eyes to allow a tracking of head motion at a frame rate of 60 Hz. The pose of the subject at the beginning of each scan serves as a reference point for prospective motion correction. Radio frequency pulses and gradients of the MR sequence are updated during the scan to keep the marker at a constant position relative to the scanner coordinate system. For highly accelerated imaging, incoherent sub-sampling of k-space in the ky-kz phase-encoding plane is achieved for each slab using a variable-density Poisson disk sampling pattern7. Multiple overlapping thin slabs are acquired with conventional TOF sequence parameters, except for k-space encoding. After data acquisition, the final volumes, $$$\mathbf{x}$$$, are obtained from the sub-sampled data, using compressed sensing reconstruction optimizing following equation with the FISTA algorithm8:
$$\min_{\mathbf{x}} \frac{1}{2} \left \lVert \mathbf{Ax}-\mathbf{y} \right \rVert_2^2 + \lambda \left \lVert \Phi(\mathbf{x}) \right \rVert_1 \, \text{,}$$
where $$$\mathbf{A}$$$ is the MR system matrix consisting of the coil sensitivity maps, sampling pattern and the Fourier transform. Sparsity is promoted in the regularization term, that is weighted by the regularization parameter , computing the l1-norm of $$$\mathbf{x}$$$ after wavelet transform ($$$\Phi(\cdot)$$$) using orthogonal Haar wavelets. A prototype sequence and reconstruction implementing the proposed method was fully integrated in a 3T clinical MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany). In-vivo experiments were performed in five healthy volunteers (two female, age 27-48 years), and the proposed method was compared to conventional TOF MRA. For both methods, the following parameters were matched: TR=21.0 ms, TE=3.5 ms, α=18°, 4 axial slabs with a FOV=220x220x24 mm3, voxel size=0.4x0.4x0.4 mm3, and a receiver bandwidth of 189 Hz/px. The conventional method was accelerated by a factor of 2.6 (PAT 3 and 24 integrated reference lines). Three subsequent scans were performed with the proposed method (acceleration: 7.2): One without motion and two scans with instructed head motion (head shake) every 10 s that was rehearsed prior to the experiments, while the motion-correction system was activated and deactivated. Compressed sensing reconstruction was performed with $$$\lambda$$$=0.002 and was terminated after 10 iterations. For evaluation, we compared acquisition time, overall image quality, and delineation of the vessels in corresponding line plots through the vessel lumen in all volumes.
Results and Discussion
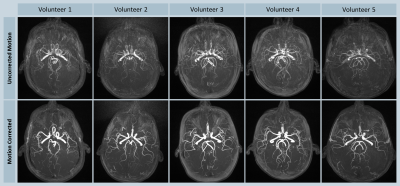
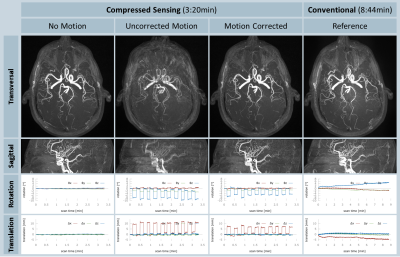
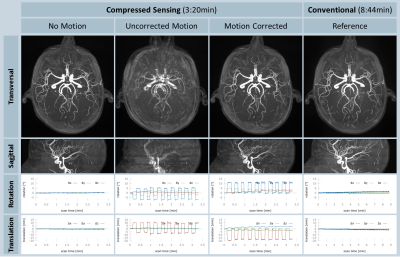
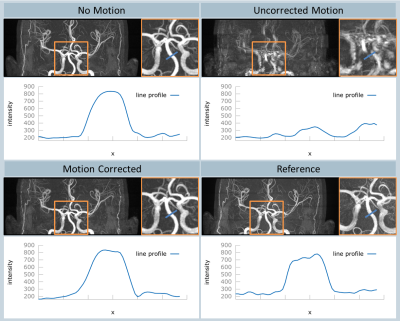
All volunteer scans finished successfully (see Figure 1). Figures 2 and 3 show the volumes as maximum intensity projection images exemplarily for two volunteers for all methods and the corresponding motion patterns as recorded for each acquisition. While maintaining the same spatial resolution, the total acquisition time was reduced from 8:44 min, using the conventional approach, to 3:20 min with the compressed sensing sequence. In the motion scans, the mean amplitude was 20.2±5.2 mm in translation (x-axis) and 13.2±2.9° in rotation (z-axis). As expected, uncompensated motion during the scan leads to severe motion artefacts in the resulting volumes. With the prospective motion-correction system, motion-induced artifacts were successfully compensated and the delineation of the vessels was restored (see Figure 4).Conclusion
The combination of compressed sensing and prospective motion correction successfully enabled a robust diagnostic image quality at a significantly reduced acquisition time in volunteers even in the presence of subject motion. This is especially important for patients suffering from acute ischemic stroke or intracranial hemorrhages where TOF has a significant clinical value. The promising results as well as the full integration of the proposed method in a standard clinical scanner enable a comprehensive evaluation in patients in the near future.Acknowledgements
We thank KinetiCor for providing the motion correction system and Ulf Gustafsson for help with setup and scanning.References
- Gonzales RG, et al., Conventional MRI and MR Angiography of Stroke, Springer Berlin 2006, pp. 115-137
- Lustig M, et al., Sparse MRI: The application of compressed sensing for rapid MR imaging, MRM 2007, 58:1182-1195
- Natsuaki T, et al., Time-Of-Flight with Sparse undersampling (TOFu): towards practical MR applications of the Compressed Sensing, ISMRM 2014, #0941
- Fushimi Y, et al., Clinical evaluation of time-of-flight MR angiography with sparse undersampling and iterative reconstruction for cerebral aneurysms, NMR in BioMed 2017, doi:10.1002/nbm.3774
- MacLaren J, et al., Prospective Motion Correction in Brain Imaging: A Review, MRM 2013, 69:621-636
- Zaitsev M, et al. Magnetic resonance imaging of freely moving objects: prospective real-time motion correction using an external optical motion tracking system. NeuroImage 2006, 31:1038–1050.
- Stalder AF, et al., Accelerating TOF MRA in Clinical Practice using Sparse MRI with Variable Poisson Density Sampling, ISMRM 2015, #3606
- Liu J, et al., Dynamic cardiac MRI reconstruction with weighted redundant Haar wavelets, ISMRM 2012, #4249
Figures