4075
MRI RF Safety of Active Implantable Medical Devices (AIMDs): Effect of Conductivity of Tissue Simulating Media on Device Model Accuracy1Micro Systems Engineering Inc., Lake Oswego, OR, United States, 2Department of ECE, University of Houston, Houston, TX, United States, 3US Food and Drug Administration, Silver Spring, MD, United States
Synopsis
The Tier 3 procedure for testing electrically long active implantable medical devices (AIMDs) for MRI RF safety involves the development of transfer function models (TFMs) of the AIMDs. Accuracy of the TFM depends on how closely the TSM mimics the tissues in which the AIMD is implanted. We find that the conductivity of the medium surrounding the lead electrode has a strong influence on the transfer function magnitude of the DUT. Therefore, TFMs of the DUT developed in a TSM whose conductivity mimics that of the in-vivo tissue that surrounds the electrode under test results in the most accurate TFM.
Introduction
The Tier 3 procedure1,2 for testing electrically long active implantable medical devices (AIMDs) for MRI RF safety involves the development of transfer function models (TFMs) of the AIMDs. The TFMs of the lead electrode relate the incident tangential E-field (Etan) along the length of the AIMD from an MRI RF coil, to the tissue RF power deposition at the lead electrode as: $$P_{RF}=A\parallel\int_{}^{}E_{tan}\left(z\right)\cdot\ TF\left(z\right)dz \parallel^{2}$$
where TF(z) is the piecewise complex transfer function of the AIMD and A is an experimentally determined constant that converts the integrated quantity to the tissue RF power deposition.
TF(z) of the device under test (DUT) is commonly measured using the piecewise excitation method3 in a tissue simulating medium (TSM). Accuracy of the TF(z) depends on how closely the electrical properties (dielectric constant, conductivity) of the TSM mimic those of the tissues in which the AIMD is implanted. There is concern that the use of TSMs with conductivity specifications provided in the ISO test specifications1,2 may result in either underestimation1 or overestimation2 of the tissue RF power deposition. Furthermore the requirement to test the lead in multiple media, when significant portions of the lead are expected to be surrounded by different media in clinical situations, imposes severe, and probably unnecessary testing requirements. In previous work4, we have shown using numerical analyses that the conductivity of the medium surrounding the lead electrode has the strongest influence on the accuracy of the TFM of the lead electrode. In this work, which is an extension of our previous work, we confirm our previous results by measuring TF(z) of a clinical AIMD.
Methods
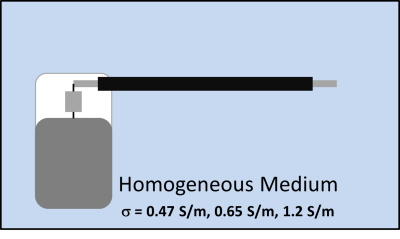
The DUT consisted of a 530 mm long bradycardia lead whose proximal electrodes were connected to an implantable pulse generator (IPG). Two sets of TF(z) of the DUT were obtained using piecewise excitation. The first set of TF(z) was obtained in homogeneous TSM (Figure 1) at 3 values of conductivity (0.47 S/m (body average conductivity)1, 0.65 S/m (myocardium conductivity) and 1.2 S/m (blood conductivity)) and relative dielectric constant of 78.
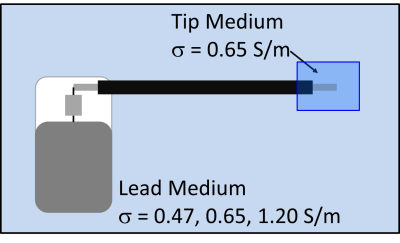
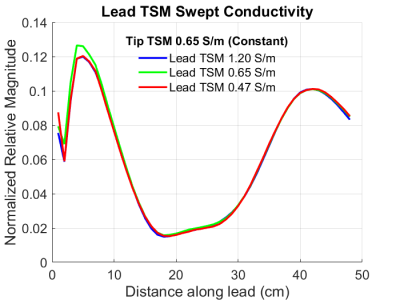
A second set of TF(z) was obtained in heterogeneous TSM (Figure 2) where the helical tip and ring electrodes of the DUT were immersed in a first TSM (tip TSM) whose conductivity was held constant at 0.65 S/m. The remainder of the DUT was immersed in a second TSM (lead TSM) whose conductivity was set to the same 3 values as with the homogeneous TSM. The relative dielectric constant of both TSMs was set to 78.
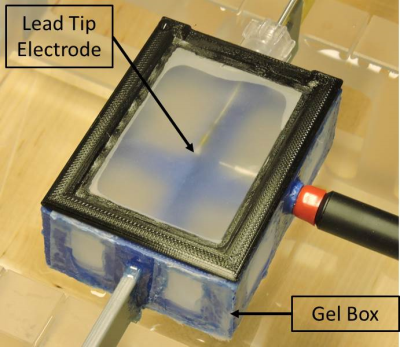
The tip TSM was implemented as a PAA gel that was placed in a mesh box as shown in Figure 3. The mesh box was then immersed in the lead TSM. The mesh material ensured electrical contact between the tip TSM and the surrounding lead TSM.
Results
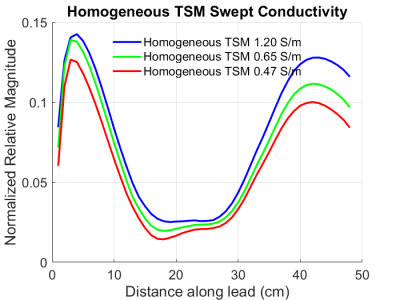
The magnitude TF(z) of the DUT measured in homogeneous TSM at the 3 conductivity values above is shown in Figure 4. The corresponding TF(z) of the DUT measured in heterogeneous TSM where the lead TSM was set to the same 2 conductivity values is shown in Figure 5.Discussion
The set of TF(z) of the DUT obtained in heterogeneous TSM (Figure 5) shows very little variation with change in the conductivity of the lead TSM. On the other hand, the set of TF(z) of the DUT obtained in homogeneous TSM (Figure 4) shows large variation of up to 28% in the high magnitude regions. These results show that the magnitude TF(z) of the DUT are sensitive to the electrical properties of the tip medium and are relatively insensitive to the electrical properties of the lead medium.
These preliminary results suggest that TF(z) of lead electrodes may need to be developed in a TSM whose conductivity is similar to the that of the tissue surrounding the lead electrode in the in-vivo situation. Furthermore, as long as the above condition is met, it may be sufficient to test the lead in an equivalent homogeneous TSM. Additional investigation is required to determine the most appropriate TSM conductivities required for specific applications.
Conclusion
The conductivity of the medium surrounding the lead electrode has a strong influence on the transfer function magnitude of the DUT, whereas the conductivity of the medium surrounding the remainder of the lead body has only a weak influence on the transfer function magnitude. Therefore, TFMs of the DUT developed in a TSM whose conductivity mimics that of the in-vivo tissue that surrounds the electrode under test results in the most accurate TFM.Acknowledgements
No acknowledgement found.References
1 ISO/TS 10974 Ed.1, Clause 10
2 ISO/TS 10974 Ed. 2, Clause 8
3 Park SM et al, JMRI 2007 Nov;26(5): 1278-85
4 Kurpad K et al, ISMRM Workshop on Ensuring RF Safety in MRI: Current Practices & Future Directions, McLean VA, Sep 28-Oct 1 2017.
Figures