4055
Magnetic resonance imaging of patients with conventional and MR-conditional pacemakers: assessment of risk and incidence1Surgical Sciences, Uppsala University, Uppsala, Sweden, 2Clinical Sciences, Lund University, Lund, Sweden, 3Cardiology, Uppsala University, Uppsala, Sweden
Synopsis
Magnetic resonance imaging of patients with conventional- and MR-conditional pacemakers should be considered and may be considered respectively, based on a case-by-case risk-benefit assessment. However, information regarding incidence of risks with respect to exposure to different electromagnetic fields is limited. Based on a literary review, risk, complications and incidence was assessed. Most common risk/complication is reed switch activation. Electrode heating is a relevant risk, with minor clinical implication. Off-label MR-examinations of MR-conditional pacemakers or abandoned leads may be considered.
Introduction
In 2013, the European Society of Cardiology published guidelines on pacing in which the recommendations classes for magnetic resonance imaging (MR) examinations performed in patients with pacemakers were Class IIa (should be considered) for MR-conditional pacemakers and Class IIb (may be considered) for conventional pacemakers, respectively1. In Germany, recent experience suggested that few hospitals performed MR-examinations on patients with pacemakers, even those labelled as MR-conditional. In response, the German Roentgen Society published clinical guidelines in 20152, and later in 2017, a joint consensus paper was published with the German Cardiac Society3. Concurrently, an international consensus paper was published by the Heart Rhythm Society, with the objective to present practical recommendations for healthcare providers for the management of patients with pacemakers who are to undergo MR-examinations4. MR-conditional pacemakers are, however, only “conditional” if the conditions as specified by the manufacturer are met. The thorax exclusion criteria, for example, can contradict patient’s thoracic MR-examinations. Furthermore, an off-label MR-examination, if clinically indicated in patients with MR-conditional pacemaker, should be defined as recommendation class IIb, i.e. may be considered. The decision to perform a MR-examination should be based on a case-by-case risk-benefit assessment1,3,4. However, information regarding incidence of risks with respect to exposure to different electromagnetic fields is limited and has not been described in detail. Thus, the intent of this work is to help healthcare professionals to manage patients with pacemakers referred to MR-examination with more detailed information regarding risk, complications and incidence, and by conceptualise this in a new comprehensive visual approach.Method
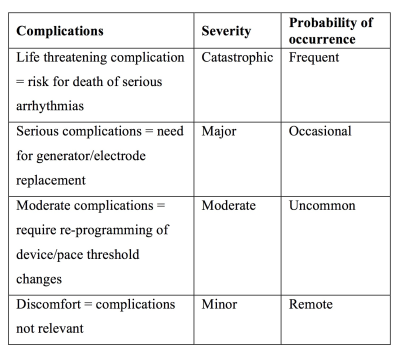
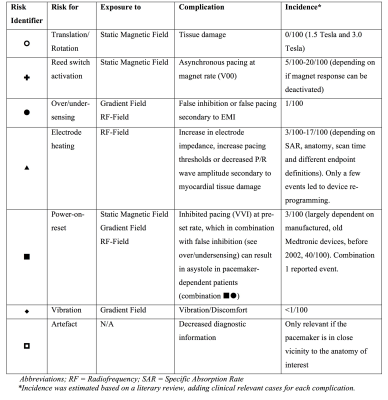
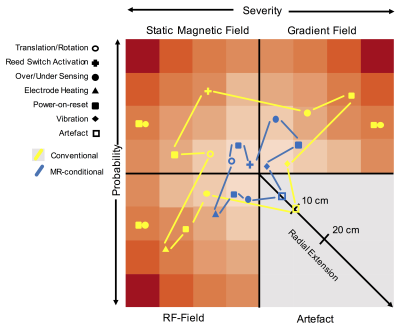
A PubMed search using the terms “pacemakers and MRI” and “MRI conditional pacemakers” was performed in November 2017. Case reports, original research- and review articles presenting patient material and/or citations to other articles were considered relevant. Conditions for MR-conditional pacemakers has been reviewed elsewhere. The assessment of risk is based on the severity and probability of a complication to occur secondary to the prevalence of a risk factor when exposed to one or more electromagnetic fields in the MRI environment. Severity is classified as; minor, moderate, major and catastrophic and Probability as; remote, uncommon, occasional and frequent (see table 1)7. Severity and probability of a given risk/complication considers the clinical impact of the complication and the incidence, estimated on data from the aforementioned literary review. It assumes that safety measures similar to those described by Nazarian8 and Russo9 are implemented before, during and after MR-examination. For MR-conditional pacemakers, common manufactural changes are considered10. The risk assessment matrix was created to visualise risk and complications. Three diagrams, each representing severity and probability for a given risk/complications are categorised by electromagnetic field exposure. The artefact is described as radial extension from the pulse generator in cm’s, disregarding electrode artefact.Results
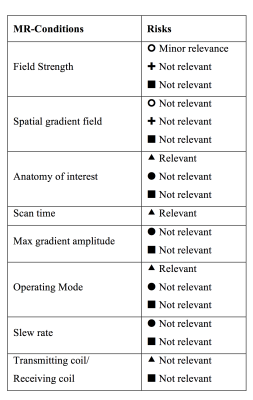
A total of 56 relevant articles were found including a total of 2800 and 700 MR-examinations of patients with conventional and MR-conditional pacemakers respectively. The risk assessment matrix is shown in figure 1 (supplementary data in table 2). MR-conditions and relevant risks for off-label use is presented in table 3.Discussion
The most common incident is reed switch activation due to exposure to the static magnetic field (asynchronous pacing, V00, at magnet rate). This should be anticipated for pacemaker without the ability to deactivate the magnet response. Other common events are power-on-reset (inhibited pacing, VVI, at pre-set rate) and electrode heating (myocardial tissue damage). Power-on-reset events were generally limited to older Medtronic pacemakers and more common during MR-examinations of brain or lumbar spine, exposing the pacemaker to high gradient magnetic fields. Several MR-examinations reporting power-on-resets used transmit/receive head coils suggesting that exposure to gradient magnetic fields is the major cause. Several events of electrode heating were concluded; however, incidence was largely dependent on endpoint limits regarding change in pacemaker parameters. Moreover, only a handful of events eventuated in re-programming of pacemaker parameters, thus the clinical implication of electrode heating can be considered minor. Specific Absorption Rate, scan time and anatomy, all related to electrode heating, are the only conditions associated to relevant, but small increase in risk for off-label use of MR-conditional pacemakers. Risks for patients with abandoned lead performing MR-examinations can be considered minor11 and artefacts are only an issue if the pacemaker is in close vicinity to the anatomy of interest. In conclusion, reed switch activation should be anticipated when deactivation is not possible. Electrode heating is a relevant risk, however with minor clinical implications. Off-label MR-examinations of MR-conditional pacemakers or abandoned leads may be considered, when following conventional pacemaker safety guidelines, with electrode heating being the most relevant risk.Acknowledgements
No acknowledgement found.References
1. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on e pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE; ESC Committee for Practice Guidelines (CPG), Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, Kirchhof P, Blomstrom-Lundqvist C, Badano LP, Aliyev F, Bänsch D, Baumgartner H, Bsata W, Buser P, Charron P, Daubert JC, Dobreanu D, Faerestrand S, Hasdai D, Hoes AW, Le Heuzey JY, Mavrakis H, McDonagh T, Merino JL, Nawar MM, Nielsen JC, Pieske B, Poposka L, Ruschitzka F, Tendera M, Van Gelder IC, Wilson CM. Eur Heart J. 2013 Aug;34(29):2281-329. doi: 10.1093/eurheartj/eht150. Epub 2013 Jun 24
2. Sommer T, Luechinger R, Barkhausen J, Gutberlet M, Quick HH, Fischbach K, et al. German Roentgen Society Statement on MR Imaging of Patients with Cardiac Pacemakers. Rofo. 2015;187(9):777-87.
3. Sommer T, Bauer W, Fischbach K, Kolb C, Luechinger R, Wiegand U, et al. MR Imaging in Patients with Cardiac Pacemakers and Implantable Cardioverter Defibrillators. Rofo. 2017;189(3):204-17.
4. Indik JH, Gimbel JR, Abe H, Alkmim-Teixeira R, Birgersdotter-Green U, Clarke GD, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm. 2017;14(7):e97-e153.
5. Fahlstrom M, Teder P, Subasic I, Aberg K, Larsson EM, Blomstrom-Lundqvist C. Pacemaker is no absolute contraindication for MRI. Lakartidningen. 2017;114.
6. Fahlstrom M, Åberg K, Bjerner T, Teder P, Olsrud J, Blomstrom-Lundqvist C, et al. MRI - a useful imaging modality even in patients with Cardiac Implantable Electronic Devices - a review of the latest developments. Magnetic Resonance Materials in Physics, Biology and Medicine. 2016;29(Issue 1 Supplement):1352-8661.
7. Department of Veteran Affairs VHA. VHA National Patient Safety Improvement Handbook, 2011. Washington, D.C., United States; 2017
8. Nazarian S, Hansford R, Roguin A, Goldsher D, Zviman MM, Lardo AC, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med. 2011;155(7):415-24.
9. Russo RJ, Costa HS, Silva PD, Anderson JL, Arshad A, Biederman RW, et al. Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. N Engl J Med. 2017;376(8):755-64.
10. Ahmed FZ, Morris GM, Allen S, Khattar R, Mamas M, Zaidi A. Not all pacemakers are created equal: MRI conditional pacemaker and lead technology. J Cardiovasc Electrophysiol. 2013;24(9):1059-65.
11. Padmanabhan D, Kella DK, Mehta R, Kapa S, Abhishek D, Mulpuru S, et al. Safety of Magnetic Resonance Imaging in Patients with Legacy Pacemakers and Defibrillators and Abandoned Leads. Heart Rhythm. 2017.
Figures