4054
Longitudinal Observation of Focused Ultrasound Induced Blood-Brain Barrier Opening of Cat Brain from 7-T Contrast-Enhanced MRIXianfeng Feng#1, Tingting He#1, Xiao Yu1, Chih-Hung Tsai2, Wen-Yen Chai3, Chao-Ting Wang1,2, Wei Xiong1, Bin Xu1, Yifeng Fan1, Hao-Li Liu*1,2, and Hsin-Yi Lai*1
1Interdisciplinary Institute of Neuroscience and Technology, Qiushi Academy for Advanced Studies, Zhejiang University, Hangzhou City, China, 2School of Medicine, Department of Electrical Engineering, Chang Gung University, Taoyuan County, Taipei City, Taiwan, 3Department of Diagnostic Radiology and Intervention, Chang Gung Memorial Hospital, Taoyuan County, Taiwan
Synopsis
Blood-brain barrier (BBB) has long been impeding the application of many therapeutic agents in treating diseases in central nervous system. Results showed that microbubble-mediated focused ultrasound can open BBB in cats noninvasively and locally. The size and duration of BBB opening can be accurately monitored by 7T MRI longitudinally. The combination of two techniques has the potential to be further applied for further clinical practice in the future.
Introduction
It has been confirmed that intravascular presence of microbubbles (MBs) with burst focused ultrasound (FUS) exposure can temporally disrupt tight-junction of cerebral capillaries[1,2] and allow increase the CNS capillary permeability (or so-called BBB opening) to facilitate brain drug delivery [3]. It is critical to confirm the consistence of exposure threshold to open BBB opening as well as safety after across various animal species preclinically prior to clinical adoption. Previous studies have shown evidences in rodents of using focused ultrasound to effectively open the BBB without brain damage in small animals and primates [3,4,5]. However, there is missing information whether middle-size animal species such as cats can have equivalent BBB-opened effect as other species did. Ultra-high field magnetic resonance imaging (MRI) provides noninvasive and high-resolution anatomical information to examine the leakage of BBB can serve as a favorable whole-brain scale permeability-changing assessing tool to monitor [6]. The aim of this study evaluates the dynamics of focused-ultrasound induced BBB opening in the cats under different ultrasound exposure levels via contrast-enhanced T1-weighted images (T1-WIs) of 7-Tesla MRI and confirmed by Evans Blue (EB) brain staining.Methods
Six cats were conducted the focused-ultrasound induced blood-brain barrier opening procedure, with the microbubbles was administered intravenously prior to ultrasound exposure and presented during ultrasound exposure (Sonovue, Bracco; 1.5µL/ kg). Three ultrasound exposure levels (estimated transcranially) of 0.6, 0.8 and 1.0 MI were applied via a 500-kHz focused ultrasound transducer, under the following exposure settings: burst length = 10 ms, pulse-repetition frequency = 1Hz, exposure time = 30 or 60s. Whole-brain contrast-enhanced T1-weighted images by a turbo spin echo (TSE) sequence (TR=2300 ms, TE=18 ms, BW=100 Hz, Voxel size: 0.5×0.5×1.0 mm3) were obtained using 7T research system (Siemens, Erlangen, Germany) before FUS, and 0.5, 3.5, 6.5 h and 1 day post-FUS. The MRI contrast-agents, Gd-DTPA (Gd-DTPA, 0.3 mmol/kg), were IV injected at every timepoints to evaluate the BBB permeability. In addition, to spatially identify the locations of BBB opening, Evans blue dye (1 ml/kg) was administered intravenously immediately following the first FUS exposure. After finishing all MRI recordings, all animals were deeply anesthetized with chloral hydrate (350 mg/kg) and sacrificed to obtained EB-stained brains. For post imaging analysis, the region of interest (ROI) selected in CE-T1WI was 0.004742 cm2 (20 pixels) per image slide. The changes of signal intensity (SI) in ROIs were depicted a long timeline.Results and Discussion
Gd-DTPA leakage at the FUS exposure site was evaluated via SI changes obtained from the subtracted T1WI between prior and post image to identify BBB-opening. Figure 1 manifests that 0.6 MI (60 s) exposure was not able to induce BBB-opening in cat brain. In addition, SI change can be obtained from 0.8 MI (30 s), but the SI change significantly decreased at 6.5h post-FUS exposure, representing that recovery of the BBB. The extreme FUS exposure up to 1.0 MI (30 s) presented a strong Gd-DTPA leakage even after 6.5h post FUS exposure, representing the BBB at the target regions is still opened at this time point. However, CE-T1WI scanned 1 day after FUS exposure (Figure 2A) did not show SI change for all exposure parameters, supporting the BBB return to intact status for all exposure level including 1.0 MI exposure. Results of EB staining (Figure 2B) and SI changes obtained from ROIs along time (Figure 3) can be well correlated from the observations showing in Fig. 1. In general, higher exposure level contributes to the increase of BBB-opening effect, whereas the exposure time change did not post significant BBB-opened scale change effect. While comparing with the previous small-animal (rodent) studies, it was observed that the threshold to open the BBB in cats (between 0.6-0.8 MI) was at the same scale but slightly higher than the previous reported level in rodents (0.5-0.6 MI) [6,7].Conclusion
We first confirmed that MB-presented FUS exposure can successfully induce temporal BBB opening in cats, and present the observation under 7T-MRI. It is also confirmed that the BBB-opened process is reversible and the BBB closure time was majorly dependent to FUS exposure level, which is consistent with the findings from other animal species. This technique, together with 7T MRI, might potentially be applied to the nonhuman primates and further clinical situations.Acknowledgements
This work was supported by grants from the Fundamental Research Funds for the Central Universities (2016QN81017) and the National Natural Science Foundation of China (81527901, 61673346, 81527901)References
[1] Lin C Y, Hsieh H Y, Pitt W G, et al. Focused ultrasound-induced blood-brain barrier opening for non-viral, non-invasive, and targeted gene delivery. Journal of Controlled Release, 2015, 212:1-9. [2] Lin C Y, Hsieh H Y, Chen C M, et al. Non-invasive, neuron-specific gene therapy by focused ultrasound-induced blood-brain barrier opening in Parkinson's disease mouse model. Journal of Controlled Release, 2016, 235:72-81. [3] Doolittle ND, Miner ME, Hall WA, et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood–brain barrier for the treatment of patients with malignant brain tumors. Cancer.2000; 88: 637–647. [4] Pardridge, W. M. Blood-brain barrier drug targeting enables neuroprotection in brain ischemia following delayed intravenous administration of neurotrophins. Adv Exp Med Biol, 2002, 513, 397-430, 2002. [5] McDannold N, Arvanitis CD, Vykhodtseva N, et al. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res., 2012, ;72:3652-63. [6] Chu PC, Chai WY, Tsai CH, et al. Focused Ultrasound-Induced Blood-Brain Barrier Opening: Association with Mechanical Index and Cavitation Index Analyzed by Dynamic Contrast-Enhanced Magnetic-Resonance Imaging. Sci Rep. 2016.6:33264. [7] McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med Biol. 2008. 34(5):834-40.Figures
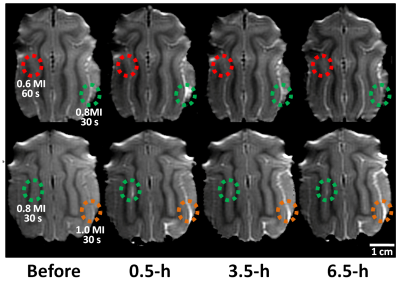
Fig1. Gd contrast T1-weighted
images at 0.5-h, 3.5-h, 6.5-h for monitoring the BBB opening in the cats‘ brain with
different acoustic pressures and exposure times of FUS.
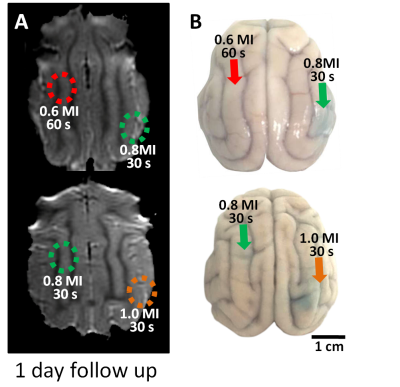
Fig2. (A) Gd
contrast T1-weighted images showed that BBB restored at 1 day post FUS in all FUS parameters. (B) EB-stained brain showed the leakage
region in the cats‘ brain.
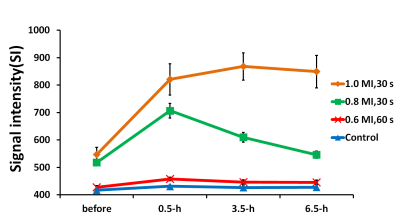
Fig3. Changes of signal intensity (SI) at the FUS exposed location in T1WIs. Error bars indicate the standard error of the mean (SEM).