4053
Monitoring High Intensity Focused Ultrasound (HIFU) ablations in real time using interventional MR Elastography (MRE)1ICube CNRS / University of Strasbourg, Strasbourg, France, 2Department of Interventional Imaging, Strasbourg university hospital, Strasbourg, France
Synopsis
This study introduces the use of interventional Magnetic Resonance Elastography (iMRE) for monitoring HIFU ablations, using the acoustic radiation force as a means for generating the shear waves necessary for MRE directly from the HIFU focus. This method allows for monitoring tissue elasticity and temperature in real time during the ablation. Its feasibility is illustrated in vivo on porcine muscle. Tissue was found to stiffen significantly (+125%) when the temperature increased, and changes in tissue stiffness were found to be irreversible. These findings suggest that tissue stiffness may be an interesting biomarker, complementary to thermal dose, to monitor HIFU ablations.
Introduction
Tissue elasticity has been shown to be a promising biomarker for monitoring thermal ablations 1-3. Interventional MR Elastography (iMRE) has been developed recently in order to address this particular challenge 3. iMRE allows computing both tissue elasticity and temperature in real time, and includes specific features such as: (1) Interventional-friendly, compact mechanical exciters that use interventional needles as source for shear waves, (2) Interactive, real-time MRE pulse sequence that allow simultaneous MR Thermometry and MRE measurements, and (3) Online reconstruction of elastograms and temperature maps in real time (~ 1 Hz). The capability of the method for monitoring MR-guided laser ablations has already been demonstrated in vivo in swine liver 3-4. In this study, the iMRE method is adapted to the monitoring of High Intensity Focused Ultrasound (HIFU) ablations. The main originality of this study is the use of the HIFU transducer as a mechanical exciter through the use of the acoustic radiation force, which generates shear waves propagating directly from the focal spot while the ablation is being performed. Feasibility is demonstrated in vivo in porcine muscle tissue.Methods
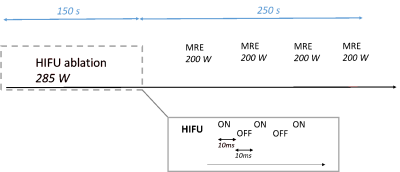
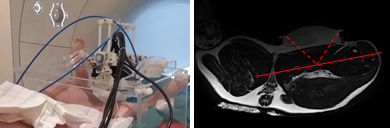
Experiments are performed using a MR-compatible HIFU system (Image Guided Therapy, France) with a 256-element transducer driven at 1 MHz. The signal transmitted to the transducer is modulated by a square wave at 50 Hz (duty cycle 50%), allowing for simultaneous heating and mechanical excitation through an oscillating pseudo-harmonic acoustic radiation force. This modulation process is depicted in Figure 1 together with the general ablation protocol used in this study. The HIFU transducer is positioned using an orientable MR-compatible structure (Figure 2). Acoustic coupling is realized through the use of a degassed water-filled balloon. MRI experiments are conducted in a 1.5T MRI system (MAGNETOM Aera, Siemens). A spoiled, interactive, interventional MRE-GRE sequence is used for encoding displacements resulting from this excitation. Main MRI parameters are: TR/TE 20ms/13ms, bipolar motion-sensitizing gradients at a frequency of 90 Hz, FOV 400 mm × 400 mm, 128 px × 128 px, 3 phase shifts, total acquisition time per slice 2.5 s. Both elasticity and temperature maps are reconstructed simultaneously using a Local Frequency Estimation-based approach and the Proton Resonance Frequency (PRF) method, respectively4. A HIFU heating phase of 150 s at an acoustic power of 285W was followed by a relaxation phase of 250 s. During the relaxation phase, isolated, short MRE measurements (2 acquisitions with opposite bipolar gradients, total acquisition time = 5 s) were performed every 60 s at lower acoustic power (200W), to avoid any additional heating. Experiments were performed in vivo in the thigh muscles of a swine, under the approval of the local ethics committee.Results
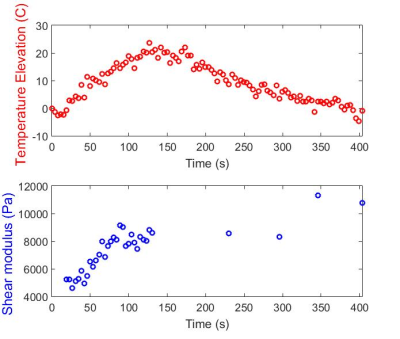
Shear waves could be clearly observed emerging from the focal region (Figure 3). Tissue shear modulus was found to increase consistently from ~4 kPa up to ~9 kPa at the end of the ablation. As expected, tissue temperature decreased back to 37°C after HIFU was turned off, while tissue elasticity remained high, with an average value of 9.5 kPa after ablation (Figure 4).Discussion
This study introduces the use of interventional MRE for monitoring HIFU ablations, using a dedicated MRE protocol that relies on the acoustic radiation force for generating shear waves. Tissue elasticity was found to increase significantly during HIFU ablation, and such increase was found to be irreversible. This study suggests that simultaneous MRE/MR Thermometry may be an interesting tool for monitoring HIFU ablations in real time. However, further investigation is needed to assess the value of tissue elasticity as a biomarker. Ongoing work is focused on comparing tissue elasticity and thermal dose to histology in order to establish the sensitivity of such biomarkers. Beyond its potential for HIFU monitoring, the method presented in this study may also be interesting for generating localized shear waves in deep-lying organs for MRE, as an alternative to external, conventional excitation systems that are associated with limitations in terms of shear wave penetration. However, further studies are needed to ensure that this excitation method complies with regulatory requirements in terms of acoustic energy that is deposited.Conclusion
These preliminary results suggest that tissue elasticity is an interesting biomarker directly related to permanent, irreversible tissue damage, as opposed to temperature. This study illustrates a new method that allows for simultaneous HIFU ablation and MRE/MR Thermometry measurements without the use of additional excitation devices.Acknowledgements
This study was partly funded by French excellence program Labex CAMI (ANR-11-LABX-0004)References
1. Mariani A, Kwiecinski W, Pernot M et al. Real time shear waves elastography monitoring of thermal ablation: in vivo evaluation in pig livers. J. Surg. Res. 2014;188:37-43.
2. Chen J, Woodrum D, Glaser K et al. Assessment of in vivo laser ablation using MR elastography with an inertial driver. Magn. Reson. Med. 2014;1:59-67.
3. Corbin N, Vappou J, Breton E et al. Interventional Magnetic Resonance Elastography for MRI-guided percutaneous procedures. Magn. Reson. Med. 2015;75(3):1110-1118.
4. Corbin N, Vappou J, Breton E et al. Interventional Magnetic Resonance Elastography for MRI-guided percutaneous procedures. ISMRM, Toronto, Canada, 2015.
Figures