4051
Model Predictive control of MRI-guided HIFU for Hyperthermia1Radiology, University Clinic of Cologne, Cologne, Germany, 2Eindhoven University of Technology, Eindhoven, Netherlands, 3Philips Research Germany, Cologne, Germany
Synopsis
Hyperthermia has been shown in clinical trials to strongly enhance therapeutic efficacy of radio- and chemotherapy. The main challenge in hyperthermia is to achieve spatially homogenous temperatures between about 41-43 oC for ca. one hour. Magnetic Resonance Imaging-guided High Intensity Focussed Ultrasound (MR-HIFU) enables heating of tissues with high spatial accuracy. We developed a new regulatory algorithm based on Model Predictive Control (MPC) for stable MR-HIFU-hyperthermia, which is designed to find effective heating patterns based on predictions of the temperature evolution in tissue. A comparison with the currently available controller is presented, detailing advantages, shortcomings and opportunities.
Introduction
Hyperthermia has been shown in clinical trials to strongly enhance therapeutic efficacy of radio- and chemotherapy1,2. Nonetheless, precise control of the spatial heat distribution is still a challenge and has been identified as one of the reasons for mixed outcomes2. Magnetic Resonance Imaging-guided High Intensity Focussed Ultrasound (MR-HIFU) allows to noninvasively induce heating with millimeter-scale accuracy under near real-time temperature feedback and is therefore the ideal technology to address this problem. Current control algorithms for MR-HIFU generally use pre-defined sonication trajectories and feedback laws with rigid gains3. While this approach works well for homogenous tissues and circular targets, it does not allow adjustment of the treatment area shape, nor to account for tissue inhomogeneity like blood vessels, and further lacks protective measures for nearby sensitive structures like nerves. To fully leverage the capabilities of MR-HIFU in these points, we have therefore developed a control algorithm which rapidly calculates the most effective heating strategy based on a thermal model and the current temperature in the target region while observing constraints on power output and maximum temperatures in protected areas. The temperature feedback is obtained via MR-imaging using Proton Resonance Frequency Shift (PRFS) thermometry. This control scheme is widely known as Model Predictive Control (MPC) and is the predominant solution to modern advanced process control problems4.Methods
Control Algorithm
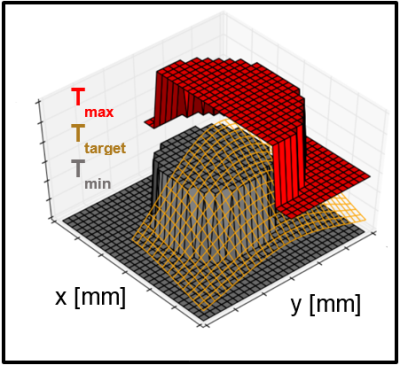
The MPC algorithm optimizes sonication patterns, aiming to heat the target tissue to a user-specified temperature range. This is done via constrained optimization: The controller uses a system of linear difference equations (LDE) to predict the evolution of temperature in response to heating. The LDE system (‘the model’) is derived from the Pennes bioheat transfer equation, describing the dependency of temperature change from diffusion, perfusion and heating. The cost function to be optimized reflects the difference between the actual temperature and the target temperature range (Figure 1). For the optimization, the algorithm varies heat delivery to a set of target points and uses the model to find the sonication pattern which will lead to the most favorable temperature distribution. The PRFS temperature readout from the MRI functions as feedback to update the controller’s thermal model.
Setup
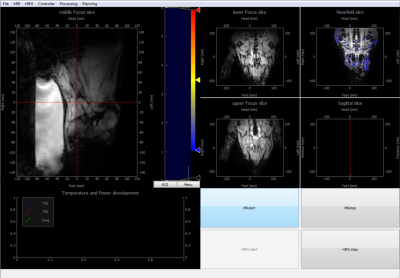
To accommodate the treatment planning tools required for this algorithm, we developed a custom MR-HIFU control software using pyMRI and pyHIFU for communication with the MR-HIFU hardware5 (Figure 2). Gurobi was used as the optimization engine6. The used hardware was a commercially available 3T MRI (Achieva®, Philips Healthcare) with an integrated HIFU table (Sonalleve®, Profound Medical).
Experiment
The controller’s performance was compared with the commercially available Sonalleve Hyperthermia algorithm. The target was a Polyacrylamide phantom. The output power was limited to 60W and the target temperature increase was set to 5K for the Sonalleve algorithm while the target temperature range was 4K to 6K increase for the MPC controller. The diameter of the target heating area was 18mm for the Sonalleve algorithm and 20mm for the MPC algorithm. The maximum beam deflection was 9mm in both cases. The thermal properties of the phantom were assumed to be similar to muscle.
Results
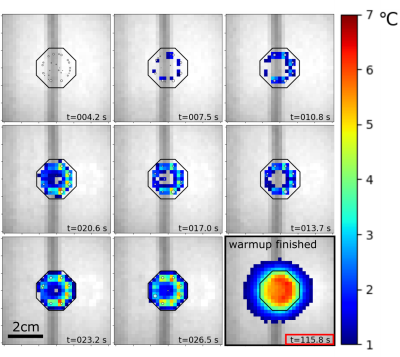
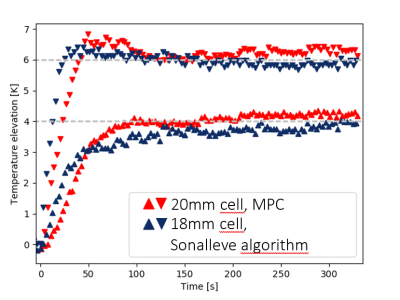
The temperature evolution resulting from an experiment with the MPC algorithm is shown in Figure 3. The performance of the two algorithms was assessed by extracting the 10th and 90th percentiles of the temperatures found in the respective ROI and plotting them versus the treatment time (Figure 4). For the MPC algorithm, we observe an elevation and stabilization of the 10th percentile to 4K by 100s, a maximum T90-temperature overshoot of 0.83 K and an average T10-T90 difference of 2.06 K after 120s. For the Sonalleve algorithm, we observe a continuous elevation of the 10th percentile over the entire time of observation, a maximum T90-temperature overshoot of 0.40 K and an average T10-T90 difference of 2.14 K after 120s.Conclusion
The experiment demonstrates the feasibility of a hyperthermia MPC algorithm as well as benefits and drawbacks of this strategy. In its current form, the controller achieves a steady state in the ROI much quicker than the commercial algorithm and produces a slightly more homogenous temperature distribution. On the other hand, it also produces a larger temperature overshoot during warmup. We expect that with a model tailored to each individual case, the control performance will become better in comparison to the current algorithm, especially in cases with inhomogeneous tissues where blood vessels provide localized heat sink effects. This will be achieved by a model identification step in the future, where thermal properties are inferred by observing the temperature evolution during and after test sonications.Acknowledgements
We thank Robert Staruch for his suggestions and helpful discussions. This work is funded by the European Union via the 7th Framework Programme.References
1. Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem B, Berard L. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncology 2010; 28(5):447-452.
2. Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Schlag PM. Hyperthermia in combined treatment of cancer. The Lancet Oncology 2002;3(8):487–497.
3. Tillander, M., Hokland, S., Koskela, J., Dam, H., Andersen, N. P., Pedersen, M., Köhler, M. High intensity focused ultrasound induced in vivo large volume hyperthermia under 3D MRI temperature control. Medical Physics 2016;43(3):1539-1549.
4. Lee, J. H. Model predictive control: Review of the three decades of development. International Journal of Control, Automation and Systems 2011;9(3):415–424.
5. Zaporzan B, Waspe AC, Looi T, Mougenot C, Partanen A, Pichardo S. MatMRI and MatHIFU: software toolboxes for real-time monitoring and control of MR-guided HIFU. Journal of Therapeutic Ultrasound 2013;1(7)
6. Gurobi Optimization Inc.. Gurobi Optimizer Reference Manual. 2016 http://www.gurobi.com. Accessed November 8, 2017
Figures