4048
Feasibility of MR guided High Intensity Focused Ultrasound (MRgHIFU) for treating recurrent gynecological tumours: comparing T2W imaging and diffusion weighted imaging (DWI) for treatment planning1CRUK Cancer Imaging Centre, Institute of Cancer Research, London, United Kingdom, 2CRUK Cancer Imaging Centre, Royal Marsden Hospital, London, United Kingdom, 3Therapeutic Ultrasound, Institute of Cancer Research, London, United Kingdom, 4Gynae-Oncology, Royal Marsden Hospital, London, United Kingdom
Synopsis
MR guided high intensity focused ultrasound (MRgHIFU) treatment plans were generated for 16 patients with recurrent gynecological tumors. Gross tumor volumes (GTV) defined on diffusion-weighted imaging using an echo-planar and turbo-spin echo technique (EPI-DWI, TSE-DWI) were approximately 20% smaller than GTV defined on T2W imaging, but did not result in sequence-dependent differences in planning target volume (PTV). PTVs were more easily defined on DWI. However, there were clinically relevant discrepancies (>4 mm) between T2-defined and DWI-defined PTV locations, worst in the phase-encode direction using EPI-DWI, that need to be accounted for if incorporating DWI into MRgHIFU treatment planning.
Background
Treatment options for recurrent gynecological malignancy are limited, particularly after radiation therapy1,2. MR guided high intensity focused ultrasound (MRgHIFU) may provide an additional treatment strategy, and its feasibility is being trialed (NCT02714621)3 using a Philips 3T Achieva MR/Sonalleve HIFU clinical system. Treatment planning is based on definition of an ellipsoid planning target volume (PTV), which encompasses gross tumor volume (GTV), but is not conformal to it. The definition of GTV on T2W imaging is challenging because tumor margins can be difficult to distinguish on a background of post-surgical or radiotherapy-induced tissue changes4,5. Diffusion weighted imaging (DWI) can improve tumor-to-normal tissue contrast6,7, but is susceptible to image distortions, particularly when using an echo-planar technique8,9. Therefore, GTV and PTV defined on DWI may be spatially misaligned with T2W images, resulting in errors in treatment prescription.Purpose
To compare the volume and spatial concordance of the GTV and PTV obtained using an echo-planar (EPI) or turbo-spin-echo (TSE) DWI technique with those from reference T2W imaging.Methods
Patients: 16 patients provided written, informed consent to participate. All had confirmed recurrent gynecological malignancy and no contra-indications to MRI. Primary tumor types were: cervix n=5, endometrium n=4, ovary n=4, vulva n=2, uterus n=1.
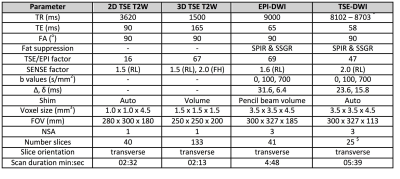
Imaging: Slice-matched 2D-T2W, distortion-corrected 3D-T2W, and EPI-DWI and TSE-DWI sequences were acquired using HIFU window and pelvic coils in combination. Sequence parameters are summarized in Table 1. T2W imaging and EPI-DWI was obtained in all 16 patients, whereas TSE-DWI was obtained in n=11 (due to sequence optimisation in n=3, and patient tolerance reasons in n=2).
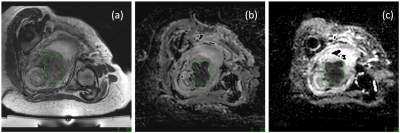
Reference T2W analysis: GTV was defined by a single observer on 2D-T2W imaging (T2-GTV) by manually drawing a ROI around tumor on every slice demonstrating it, using in-house software (Adept, ICR, London), and multiplying summated ROI areas by slice thickness. 2D- and 3D-T2W images exported to the Sonalleve treatment planning system were then used to define PTV (T2-PTV), to conform as closely as possible to the tumor region defined by the GTV. The AP, LR and FH co-ordinates of the PTVs central point (obtained by using the software’s pixel information tool) were used to determine the location of each T2-PTV.
DWI analysis: GTV and PTV were subsequently defined on EPI-DWI and TSE-DWI by the same observer, without reference to the T2W images. PTV and GTV were each defined on apparent diffusion coefficient (ADC) maps calculated from the b=100 and b=700 s/mm2 data. Adept software was used to define GTV, and treatment planning software to define PTV.
Method comparison: Volumes of DWI-GTVs and DWI-PTVs were compared graphically with T2-GTVs and T2-PTVs for all patients, and using paired samples t-tests for n=11 with matched imaging data. Discrepancies in DWI-PTV and T2W-PTV positions were calculated as the magnitude difference values between each set of AP, LR and FH co-ordinates.
Results
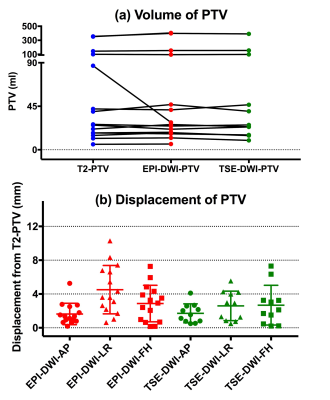
T2-PTV was always larger than T2-GTV: mean±SD percentage difference = 25.9±13.5% (range: 4.4-56.7%).
GTV defined on DWI was approximately 20% smaller than GTV defined on T2W imaging (mean±SD percentage difference: T2-GTV vs EPI-DWI-TSE = 22.5±20.6%, T2-GTV vs EPI-TSE-DWI = 19.1±17.8%). These differences achieved statistical significance in n=11 with matched imaging data (T2 vs EPI-DWI: p=0.01, T2 vs TSE-DWI: p=0.006).
Sequence-dependent differences in GTV did not result in sequence-dependent differences in volumes of PTV (T2-PTV and EPI-DWI-PTV: p=0.37, T2-PTV and TSE-DWI-PTV: p=0.80). However, discrepancies in PTV locations were seen (Figures 1,2). These were worst for EPI-DWI in the LR (phase-encode) direction (maximum/mean±SD: 10.3/4.5±2.9 mm), compared to 5.5/2.6±1.7 mm for TSE-DWI. Discrepancy in the HF (slice-encode) direction was 7.3/2.9±2.2 mm for EPI-DWI, and 7.3/2.7±2.4 mm for TSE-DWI. There was least error in the AP (frequency-encode) direction (EPI-DWI: 5.3/1.6±1.3 mm, TSE-DWI: 4.1/1.7±1.1 mm).
Discussion and Conclusion
Using DWI for tumor outlining instead of T2W imaging resulted in significantly smaller GTVs, but not significantly smaller PTVs. PTVs were easier to define on DWI. However, displacements in PTV meant that some treatment cells would have been delivered inaccurately if PTV definition were based on DWI alone. A spatial discrepancy of ±4 mm equates to 2 incorrectly positioned 4 mm diameter treatment cells in every line of that imaging plane (one under-treated, one over-treated). Our data show that this clinically relevant degree of error occurred in the phase-encode direction in approximately half of the patients using EPI-DWI. Whilst TSE-DWI reduced error in the phase-encode direction, discrepancies of up to 7 mm were still seen in the foot-head direction. Therefore, the improved contrast afforded by DWI assists tumor outlining, but additional reference to T2W imaging is advised when using it for treatment planning of MRgHIFU.Acknowledgements
CRUK and EPSRC support to the Cancer Imaging Centre at ICR and RMH in association with MRC & Department of Health C1060/A10334, C1060/A16464. NHS funding from the NIHR Research for Patient Benefit programme (PB-PG-0815-20001), and to the NIHR Biomedical Research Centre, the Clinical Research Facility in Imaging and the Cancer Research Network. We would also like to acknowledge the support of Philips Healthcare and the Focused Ultrasound Foundation.References
1. Ang, C., et al., Exenterative surgery for recurrent gynaecological malignancies. Cochrane Database Syst Rev, 2014(2): p. CD010449.
2. Chiva, L.M., et al., Surgical treatment of recurrent cervical cancer: state of the art and new achievements. Gynecol Oncol, 2008. 110(3 Suppl 2): p. S60-6.
3. https://clinicaltrials.gov/ct2/show/NCT02714621. MR-HIFU for Recurrent Gynaecological Cancer (HIFU-Gynae). 2017 [cited 2017 20/09/2017].
4. Brown, M.A. and H.R. de Abreu, MR imaging of malignant uterine disease. Magn Reson Imaging Clin N Am, 2006. 14(4): p. 455-69, v-vi.
5. Hameeduddin, A. and A. Sahdev, Diffusion-weighted imaging and dynamic contrast-enhanced MRI in assessing response and recurrent disease in gynaecological malignancies. Cancer Imaging, 2015. 15: p. 3.
6. deSouza, N.M., A. Rockall, and S. Freeman, Functional MR Imaging in Gynecologic Cancer. Magn Reson Imaging Clin N Am, 2016. 24(1): p. 205-22.
7. Manoharan, D., et al., Diffusion weighted imaging in gynecological malignancies - present and future. World J Radiol, 2016. 8(3): p. 288-97.
8. deSouza, N.M., et al., Distortion correction of echo-planar diffusion-weighted images of uterine cervix. J Magn Reson Imaging, 2016. 43(5): p. 1218-23.
9. Downey, K., et al., Comparison of optimised endovaginal vs external array coil T2-weighted and diffusion-weighted imaging techniques for detecting suspected early stage (IA/IB1) uterine cervical cancer. Eur Radiol, 2016. 26(4): p. 941-50.
Figures